Detection or Diagnosis of Excess Aluminum in Soils
As discussed earlier, soil aluminum can exist in many different pools, and its reactions within the soil solution are also quite intricate. It is generally accepted that the activity of monomeric hydroxyaluminum species should be a good predictor of aluminum toxicity for a given plant species if (a) the aluminum absorption by plants is small relative to the quantity of toxic aluminum species in the soil solution such that the solution activity remains virtually constant as the plant grows (steady-state condition) or (b) any decrease in the activity of toxic aluminum species is readily compensated for by solid phase aluminum or nontoxic aluminum in solution (equilibrium condition). In reality, these conditions are hardly met, thus solution activity (intensity factor) and an estimate of the aluminumbuffering capacity (capacity factor) are required to evaluate or predict the toxicity of soil aluminum.Extractable and Exchangeable Aluminum
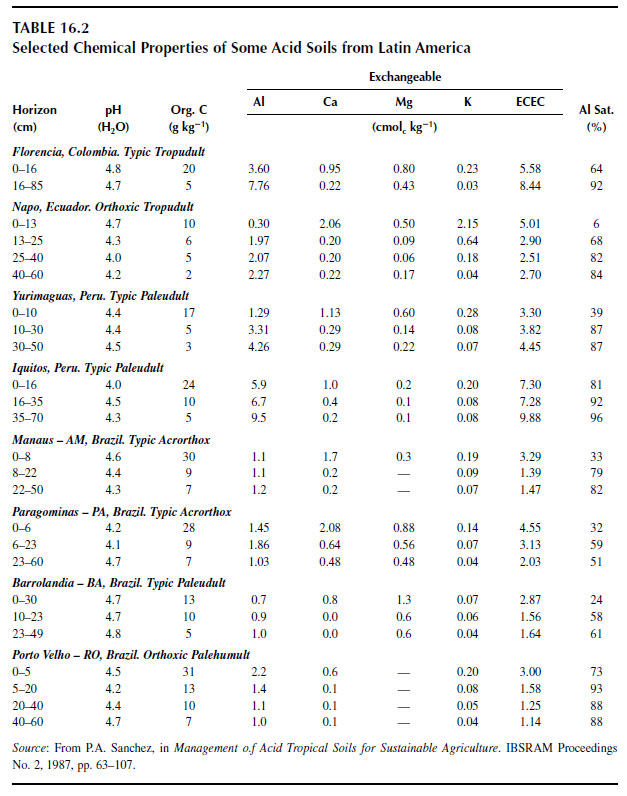
Different methods have been used to extract solid-phase aluminum, which presumably correlates well with aluminum phytotoxicity (299). An unbuffered solution of 1 M KCl is commonly used to extract the fraction of aluminum (often referred to as 'exchangeable'), which is presumably held by negative charges on the soil surface. When exchangeable aluminum is expressed as a percentage of the effective cation exchange capacity (ECEC), it is referred to as the aluminum saturation percentage. Table 16.2 lists values of exchangeable aluminum and aluminum saturation percentage for some acid soils from Latin America (300). The amount of aluminum extracted by neutral salts, such as 1 M KCl or 0.01M CaCl2, however, varies with extraction time, concentration of the extracting solution (301), and with the number of successive extractions (302).Other solutions such as 1M NH4Cl, 0.01 M CaCl2, or 0.01 M Ca(NO3)2 have also been used to extract aluminum. There are indications that aluminum extracted with 0.01 M CaCl2, an extractant that mimics the ionic strength (and composition) of highly weathered acid soils, correlates well with the free Al3+ activity in soil solution and with aluminum phytotoxicity (303-304).
Also, 0.5 M CuCl2 and 0.33 M LaCl3 have been used to extract organically bound aluminum (284,305). Copper reacts strongly with carboxylate sites that bind aluminum and can readily replace aluminum bound to the solid organic matter. Lanthanum is less effective than copper, but more effective than potassium, in displacing organically bound aluminum (306).
Despite potential difficulties in extracting toxic forms of aluminum with neutral salt solutions, exchangeable aluminum and aluminum saturation percentage have been used extensively as an indicator of aluminum toxicity in acid soils and in estimating the lime requirement (307). Growth of many plants in acid soils was reduced by 50% or more compared to growth in limed soil when the soil aluminum saturation was >60% (307). As for lime requirement, it is generally accepted that the amount of CaCO3 required to neutralize toxic aluminum can be estimated as follows:
The CaCO3 requirement (t ha-1) = K x exchangeable aluminum (cmolC kg-1)
where K ranges from 1.5 to 3.0 and averages 2.0 (307). Often K is>1 to partly account for the fraction of aluminum that is not extracted by KCl. On the other hand, as pointed out by Adams (279), the critical aluminum-saturation percentage, above which relative plant growth would be restricted by 10% or more, varies markedly with soils and crops. For example, the critical aluminum saturation for soybean was about 20 to 25% for Ultisols in Alabama and North Carolina (308-310). It was about 6% for an Ultisol in South Carolina (308), 5% for a Spodosol in Florida (311), and 30% for an Oxisol in Brazilian Amazon (312). As for different crops, the critical aluminum saturation was 4 to 5% for alfalfa, white clover, tall fescue (Festuca arundinacea Schreb.), and sericea lespedeza (Kummerowia striata Schindl., formerly Lespedeza striata) (313,314). It was 40 to 50% for corn grown on three Ultisols in North Carolina (315), 1 to 8% for six Ultisols in Georgia (316) and 30% for an Oxisol in Brazil (312). Similarly, Adams and Moore (317), using the elongation rate of cotton taproot as an indicator of aluminum toxicity, found that the critical aluminum saturation was 2% in the Bt2 horizon of one soil but more than 56% in the Bt1 of another soil in Alabama. For peanut (Arachis hypogaea L.), the critical aluminum saturation was 60% (312). Evidently, additional and perhaps better methods for identifying the toxic aluminum forms are needed.
 |
Soil-Solution Aluminum
Soil solution can be collected by several techniques, such as zero-tension lysimeters (in situ field sampling), column displacement with a miscible liquid, or high-speed centrifugation with or without a heavy liquid that is immiscible with water (laboratory sampling) (299,318). These techniques, however, are time consuming and often require high skills and care (in terms of pH changes due to CO2 loss, and contamination) especially when aluminum concentrations are at micromolar levels.Once in solution, be it soil solution or dilute neutral salt extracts, soluble aluminum can be quantified readily using atomic absorption (preferably flameless) spectroscopy or inductively coupled plasma emission spectroscopy. Alternatively, total soluble aluminum can be measured colorimetrically after forming a colored complex with an organic agent (319).
The separation of total soluble aluminum into different forms (speciation) is more involved, and many techniques have been proposed, which can be grouped into three main categories: (a) analytical separation of various aluminum fractions based on differential reaction kinetics with complexing agents or the physico-chemical separation of aluminum fractions based on size and charge; (b) computational differentiation of aluminum species from an analytically determined ‘total’ aluminum fraction, using a thermodynamically based geochemical speciation model with mass balance constraints (320); and (c) combination of one or more analytical techniques with a geochemical speciation model (321).
The most common timed spectrophotometric methods for aluminum determination include 8-hydroxyquinoline (HQ) and pyrocatechol violet (PCV) (322–325). James et al. (322) used a 15 s reaction with HQ buffered at pH 5.2, followed by extraction into butyl acetate, as a method for measuring monomeric aluminum species; a 30-min reaction would measure the total soluble aluminum. The PCV method requires a longer reaction time (approximately 20 min as suggested by Menzies et al. (325)) to complex completely with monomeric aluminum; thus, it is more suitable for an automated procedure.
Aluminum fractionation methods based on size or charge include dialysis, ultrafiltration, sizeexclusion chromatography, ion chromatography, capillary zone electrophoresis, and C-18 reversephase chromatography (299). Soluble aluminum can also be measured indirectly by reacting it with F-, then measuring the unreacted free F- with an ion-elective electrode (326). A quantitative 27Al NMR method is often preferred for the measurement of the ‘Al13’ polymer (327).




