Plant Mechanisms of Aluminum Avoidance
Based on chemical analysis of aluminum in root sections, Horst et al. (189) showed that the root tips of an aluminum-tolerant cultivar of cowpea (Vigna unguiculata Walp.) had a lower aluminum concentration than those of an aluminum-sensitive cultivar, suggesting that reduced aluminum absorption into the root tip was responsible for its higher aluminum tolerance. Using direct measurement of aluminum with atomic absorption spectrophotometry or ion chromatography, Rincon and Gonzales (190) showed that aluminum content was 9 to 13 times greater in the 0-to-2-mm root tips of an aluminum-sensitive wheat cultivar than in an aluminum-tolerant cultivar. Similar results were reported by Delhaize et al. (191), who showed using x-ray microanalysis that aluminum-sensitive wheat root apices accumulated 5 to 10 times greater aluminum than aluminum-tolerant root apices.These results indicate that aluminum exclusion occurs in several plant species. Possible mechanisms of aluminum avoidance include: (a) root avoidance response, (b) organic acid release, (c) exudation of phosphate, (d) exudation of polypeptides, (e) exudation of phenolics, (f) alkalinization of rhizosphere pH, (g) binding to mucilage, (h) binding to cell walls, (i) binding to external face of membrane, and (j) interactions with mycorrhizal fungi.
Avoidance Response of Roots
Classic avoidance response of roots to aluminum toxicity was shown by research (149) in which corn roots curved away from aluminum applied to one side of root. Also, aluminum toxicity killed cells in the corn root apical meristem, and Boscolo et al. (155) speculated that this phenomenon would result in loss of apical dominance and greater lateral root growth into environments with lower aluminum levels. Interestingly, taproots of corn cv. SA-6 and soybean cv. Perry did not penetrate much into an aluminum-toxic subsoil layer, although lateral root lengths increased in the nontoxic top soil layer (192). However, although increased lateral root growth in topsoil layers could help to maintain crop yields in areas with acid subsoils, under drought conditions, lack of root growth into deeper layers could limit water uptake.Organic Acid Release
Considerable evidence supports organic acid release as a mechanism of aluminum avoidance in plants (179,188,193,194). Hue et al. (195) used elongation of cotton (Gossypium hirsutum L.) taproots as a measure of aluminum toxicity to document the aluminum detoxification effect of several low-molecular-weight organic acids or anions. The relative ameliorative capacity of the organic acids followed closely the stability constants of the aluminum-organic acid complexes in the order:Citric>Oxalic>Tartaric>Malic>Acetic
The formation of stable rings (5-, 6-, and to a lesser extent 7-membered structures) between aluminum and organic anions or molecules seems to be responsible for the detoxification (195). Structure of an aluminum-citrate complex is shown below.
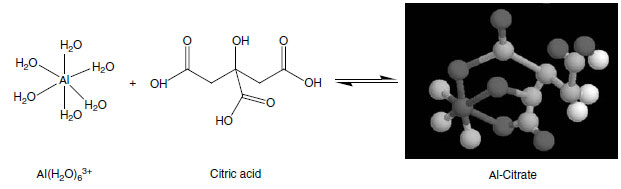 |
The first evidence of aluminum-induced root exudation of an organic acid was identified in snapbean, in which an aluminum-tolerant cultivar exuded ten times as much citrate as an aluminum- sensitive cultivar in the presence of aluminum (196). Aluminum-induced root release of malate was characterized thoroughly in wheat by Delhaize and co-workers (197-200). They showed that exposure of an aluminum-tolerant genotype to 10 µM Al induced malate exudation from roots within 15 min. Wheat root apices contained sufficient malate for excretion for over 4 h (198). After 24 h of exposure to 100 µM Al, de novo synthesis of malate was demonstrated by measuring 14C incorporation into malate (199). The efflux of malate from root apices was electroneutral, because it was accompanied by an efflux of K+ (198). Evaluating 36 wheat cultivars, Ryan et al. (200) showed a significant correlation between relative tolerance of wheat genotypes to aluminum and amount of malate released from root apices. Other researchers have argued against the effectiveness of malate exudation on alleviating aluminum toxicity because of rapid degradation by soil microorganisms (201) and the low concentrations and relatively weak chelating ability of malate for aluminum (202).
Other plant species have been shown to exude organic acids in response to aluminum stress. Aluminum-tolerant corn genotypes exuded higher concentrations of citrate (203). An aluminumtolerant tree species, Senna tora Roxb. (formerly Cassia tora), exuded citric acid after 4 h of exposure to 50 µM Al (204). In rye, after 10 h of exposure to 10 µM Al, increased activity of citrate synthase (CS) occurred along with increased citrate secretion (205). In all soybean genotypes, citrate exudation increased within 6 h of aluminum exposure; however, only citrate efflux in aluminum- tolerant genotypes was sustained for an extended time period (206). A positive correlation was found between citrate in root tips of soybean and aluminum tolerance (206). The aluminumaccumulating plant, buckwheat (Fagopyrum esculentum Moench), was found to exude oxalate, a strong aluminum chelator (207). Taro (Colocasia esculenta Schott), a tropical root crop that is not an aluminum accumulator, also exuded oxalate from roots in response to aluminum (208). Aluminum-resistant mutants of Arabidopsis thaliana constitutively released higher concentrations of citrate or malate compared to the wild type (209). A mutant carrot (Daucus carota L.) cell line that solubilized phosphate from aluminum phosphate exuded citrate from roots (210). This cell line had a greater activity of mitochondrial CS and a lower activity of a cytoplasmic enzyme, NADPspecific isocitrate dehydrogenase (NADP-ICDH), involved in citrate degradation (211,212).
Anion channels are involved in the aluminum-activated exudation of organic anions. Using electrophysiology to measure current passing across whole apical cells of wheat roots, Ryan et al. (213) showed that 20 to 50µM Al activated an anion channel. Genotypic differences were found with the aluminum-induced currents across protoplasts from the aluminum-tolerant wheat genotype occurring more frequently and being sustained for a longer period of time than those from the aluminumsensitive genotype (214). Using subtractive hybridization of cDNAs from near-isogenic lines of aluminum-sensitive and aluminum-tolerant wheat, Sasaki et al. (215) found greater expression of a gene that cosegregated with aluminum tolerance. Heterologous expression of this gene, named ALMT1 (aluminum-activated malate transporter), in Xenopus oocytes, rice, and cultured tobacco cells conferred an aluminum-activated malate efflux, and enhanced the ability of tobacco cells to recover from 18 h of exposure to 100 µM AI (215). Transgenic barley cultivars with the ALMT1 transgene showed increased malate effux and increased root grwoth at concentrations up to 12 µM AI (216).
Another means of increasing aluminum tolerance in plants is to increase synthesis as well as exudation of organic acids. De la Fuente et al. (217) overexpressed a CS gene from the bacterium, Pseudomonas aeruginosa Migula, in the cytoplasm of transgenic tobacco and found increased citrate levels within roots, increased citrate efflux, and increased root elongation in the presence of≥100 µM Al. However, Delhaize et al. (218) were unsuccessful in repeating this work (217), and they suggested that the activity of P. aeruginosa cytoplasmic CS in transgenic tobacco is either sensitive to environmental conditions, or that the improved aluminum tolerance observed by de la Fuente et al. (217) was due to other factors. Koyama et al. (219) overexpressed a mitochondrial CS gene, isolated from carrot, in Arabidopsis thaliana and found increased CS activity, increased excretion of citrate, and slightly increased amelioration of aluminum toxicity based on root elongation at pH 5.
Tesfaye et al. (220) overexpressed genes for nodule-enhanced forms of the enzymes that catalyze malate synthesis, phosphoenolpyruvate carboxylase and malate dehydrogenase in alfalfa (Medicago sativa L.). They found increased enzyme activities, increased root exudation of organic acids (citrate, oxalate, malate, succinate, and acetate), and increased root elongation in the presence of 50 to 100 µM Al. However, such root exudation represented a drain of plant resources, and transgenic lines had reduced biomass compared to untransformed control plants when grown at soil pH 7.25. In acid soils, however, transgenic alfalfa had 1.6 times greater biomass than untransformed control plants.
Although abundant evidence exists for aluminum-induced organic acid excretion as a mechanism of aluminum tolerance, other mechanisms probably exist. Ishikawa et al. (221) found no correlation between species or within species for organic acid exudation and aluminum tolerance. Similarly,Wenzl et al. (222) reported that the greater aluminum tolerance of signalgrass (Urochloa decumbens R.D. Webster, formerly Brachiaria decumbens) relative to ruzigrass (Urochloa ruziziensis Crins, formerly Brachiaria ruziziensis) was not due to greater exudation of organic acids.
Exudation of Phosphate
Root apices of an aluminum-tolerant genotype of wheat exuded phosphate as well as citrate in response to aluminum exposure (223). Pellet et al. (223) speculated that phosphate release contributed to aluminum tolerance in wheat. In contrast, no major differences in phosphate release were found among near-isogenic lines of wheat that differed in aluminum tolerance (224).Exudation of Polypeptides
Aluminum-resistant lines of wheat exuded an aluminum-induced 23 kDa polypeptide (225). This polypeptide, synthesized de novo in response to aluminum, binds aluminum, and cosegregates with the aluminum-resistant phenotype in F2 populations (225,226). The gene encoding this polypeptide still needs to be isolated.Exudation of Phenolics
Phenolics are aromatic secondary metabolites of plants (e.g., quercetin, catechin, morin, or chlorogenic acid) that can bind aluminum (67,227). Silicon ameliorates aluminum toxicity in some plants (228, 229). In an aluminum-resistant corn cultivar, silicon and aluminum triggered the release of phenolic compounds (e.g., catechol, catechin, and quercetin) up to 15 times the release by plants not pretreated with silicon (230). However, the binding capacity of many of these phenolic compounds for aluminum is greater at pH 7 than at pH 4.5 (227).Alkalinization of Rhizosphere
The solubility of aluminum is dependent on pH; as pH rises above 5.0, precipitation of aluminum as Al(OH)3 increases (Figure 16.1). An aluminum-tolerant wheat cultivar grown in a nutrient solution increased the pH, whereas an aluminum-sensitive cultivar lowered the solution pH (231). Foy et al. (231) proposed that aluminum tolerance is associated with plant-induced alkalinization of pH. However, rhizosphere pH associated with apical root tissues did not appear to be a primary mechanism of differential aluminum tolerance in wheat. The root apex of an aluminum-tolerant wheat genotype had only a slightly higher rhizosphere pH in the presence of aluminum than an aluminumsensitive genotype, resulting in a 6% decrease in free Al3+ activity (121). Yet the aluminum-tolerant wheat genotype had 140% greater relative root elongation compared to the aluminum-sensitive genotype, indicating that rhizosphere pH did not play a major role in differential aluminum tolerance (121). In contrast, Degenhardt et al. (232) reported that aluminum exposure induced a doubling in net H+ influx at the root tip of an aluminum-resistant Arabidopsis mutant relative to the wild-type, increasing pH by 0.15 units. Although the pH difference was small, solution pH maintained at 4.5 was shown to increase Arabidopsis root growth relative to that at pH 4.4.Binding to Mucilage
Horst et al. (233) reported that mucilage from root tips of cowpea had a high binding capacity for aluminum and that removal of this mucilage resulted in greater inhibition of root elongation by aluminum. They proposed that mucilage served to protect the apical meristem against aluminum injury. Similarly, Brigham et al. (234) showed that removal of snapbean mucilage (including root border cells) resulted in reduced root elongation and greater aluminum accumulation in root tips as shown by lumogallion staining. Pan et al. (777) demonstrated that the presence of mucilage and border cells in wheat reduced aluminum injury to root meristems, as shown by a greater mitotic index. In contrast, Li et al. (235) found that although mucilage from corn root apices binds strongly to aluminum, the presence or absence of mucilage did not affect aluminum-induced inhibition of root elongation.Binding to Cell Walls
Some researchers observed that root cation exchange capacity (CEC) of Al-tolerant genotypes were lower than that of aluminum-sensitive ones (236); however, other researchers found no such correlation (237,238). Interestingly, a transgenic potato overexpressing PME exhibited greater activity of PME (which should result in more free carboxylic groups in cell walls), greater aluminum accumulation in root tips, and greater sensitivity to aluminum as shown by aluminum-induced callose formation and inhibition of root elongation (108). These results suggest that genotypic differences in number of negatively charged binding sites in the cell wall could result in differential aluminum tolerance.Interestingly, overexpression of WAK1 in arabidopsis conferred increased aluminum tolerance as shown by increased root elongation in the presence of aluminum (89). Sivaguru et al. (89) speculated that WAKs could interact with cell wall components such as callose or pectins, alleviating aluminum toxicity. Alternatively, they speculated that the cytoplasmic kinase domain could be cleaved off from WAKs and participate in cytoplasmic aluminum response pathways.
Binding to External Face of Plasma Membrane
Among five plant species differing in aluminum tolerance, the zeta potential (i.e., an estimate of plasma membrane surface potential) was higher (membrane surface less negative) in aluminumresistant plant species than in sensitive ones (239). Wagatsuma and Akiba (239) hypothesized that aluminum-sensitive plant species had more negative charges on the plasma membrane, resulting in greater aluminum-binding to its surface. Similarly, Ishikawa and Wagatsuma (240) pretreated protoplasts of four plant species with aluminum for 10 min followed by a hypotonic aluminum-free solution. They found that protoplasts from aluminum-sensitive species exhibited greater leakage of K+ and proposed that aluminum binding to plasma membrane induced greater rigidity, reduced extensibility, and increased leakage under hypotonic conditions. In contrast, Yermiyahu et al. (241) found that the surface-charge density of vesicles isolated from an aluminum-sensitive wheat cultivar was 26% more negative than those from an aluminumtolerant wheat cultivar. However, they (241) argued that this small difference in surface-charge density did not account for the large difference in sensitivity to aluminum (50%).Interactions with Mycorrhizal Fungi
Conflicting reports occur in the literature with a few researchers finding negative or no effect of mycorrhizal colonization on host-response to aluminum toxicity (242-245) and a greater number showing a beneficial effect of colonization with either ectomycorrhizal (ECT) (246,247) or arbuscular mycorrhizal fungi (AMF) (248-250). Host response to aluminum toxicity depended on the species of ECT (242) or AMF (243). Scots pine (Pinus sylvestris L.) colonized by an aluminumsensitive ECT fungus (Hebeloma cf. longicaudum Kumm. ss. Lange) exhibited decreased shoot and root biomass compared to nonmycorrhizal plants in the presence of 2500 µM Al (242). In contrast, Scots pine colonized by an aluminum-tolerant ECT fungus (Laccaria bicolor Orton) had greater shoot and root biomass, greater shoot P, and lower shoot aluminum compared to nonmycorrhizal plants in the presence of 740 µM Al (242). Similarly, only five of eight isolates of AMF increased growth of switchgrass and reduced foliar Al concentrations in an acid soil (243).Pitch pine (Pinus rigida Mill.) colonized with the ECT fungus, Pisolithus tinctorius Coker and Couch, had greater shoot and root biomass at 50 to 200 µM Al than noninoculated plants (246). Colonization of white pine (Pinus strobus L.) with the ECT fungus, P. tinctorius, resulted in greater shoot dry weight, height, and needle length relative to nonmycorrhizal seedlings at aluminum levels ≥460 µM (247). Schier and McQuattie (247) attributed the beneficial effects of ECT fungi to reduced aluminum concentrations and higher phosphorus concentrations in needles.
Colonization of switchgrass (Panicum virgatum L.) with the AMF, Glomus occultum Walker, resulted in higher total shoot biomass at 500 µM Al as well as lower tissue aluminum and higher calcium concentrations (248). In an aluminum-sensitive barley cultivar, colonization with the AMF, Glomus etunicatum Becker and Gerdemann, resulted in greater shoot biomass and greater P concentrations in shoots and roots at 600 µM Al (249). Colonization of tissue-cultured banana (Musa acuminata Colla) with the AMF, Glomus intraradices N.C. Schenck & S.S. Sm., increased shoot dry weight, water uptake, and nutrient uptake and decreased aluminum content in roots and shoots (250). Apparently, one of the benefits of either ecto- or endomycorrhizal colonization is to ameliorate the detrimental effects of aluminum toxicity on root growth and nutrient or water uptake.
Aluminum has toxic effects also on mycorrhizal fungi, adversely affecting the quality and quantity of mycorrhizal colonization (243,251). Differences in response to aluminum have been found between ECT fungal species (243). Also, genotypic differences within an ECT fungal species have been found in response to aluminum. For example, isolates of ECT fungus, P. tinctorius, from old coal-mining sites (pH 4.3, 12.1mM Al) exhibited greater aluminum tolerance based on mycelial mass at≥440 µM Al than isolates from rehabilitated mine sites (pH 4.9, 800 µM Al) and those from forest sites (pH 4.3, 220 µM Al) (252). Strains of the ECT fungus, Suillus luteus Gray, that differed in aluminum sensitivity were inoculated on Scots pine, and the extramatrical mycelia developed by the aluminum-resistant strain were more abundant in the presence of aluminum compared to those of the aluminum-sensitive strain (251). Scots pine seedlings colonized by this aluminum-tolerant ECT strain in the presence of aluminum had greater shoot heights compared to noninoculated seedlings (251).
Cuenca et al. (253) showed that the tropical woody species, Clusia multiflora Knuth., inoculated with AMF accumulated less aluminum in roots; instead aluminum was bound to the cell walls of the fungal mycelium and in vesicles. Using 27Al-NMR, aluminum was found to be taken up and accumulated into polyphosphate complexes in the vacuole of the ECT fungus, Laccaria bicolor Orton (254). Martin et al. (254) suggested that sequestration of aluminum in polyphosphate complexes could help to protect mycorrhizal plants against aluminum toxicity. An aluminum-adapted strain of an ECT fungus, Suillus bovines Kuntze, had a shorter average chain length of mobile polyphosphates and greater terminal phosphate groups (255). Gerlitz (255) proposed that this change increased binding and detoxification of polyphosphates to aluminum. A good review of possible aluminum tolerance mechanisms in ECT is found in Jentschke and Godbold (256).




