Determining the Titer of A Bacterial Virus (Bacteriophage)
As we have learned, viruses are unable to multiply except inside of living cells, human or bacterial. Like other viruses, those that prey on bacteria (bacteriophages) attach to the host cell wall and insert themselves into the cell. There, one of two events may take place. In the first, the viral nucleic acid takes over the metabolic machinery of the cell, directing it to produce new viral particles. The result is eventual death and lysis (rupture) of the bacterium, with the release of many new virions that search for intact bacterial cells to infect. This process is known as the lytic cycle. In the second event, known as lysogeny, the viral nucleic acid becomes integrated into the host cell chromosome, replicating with it each time. Lysogenic bacteriophage do not harm the host cell until some process causes it to be awakened and initiate the lytic cycle. Several human pathogens, such as the diphtheria bacillus, are lysogenized by viruses whose DNA directs the synthesis of toxins that are harmful to the human host.In this experiment we will study the coliphage T2, which is a bacteriophage lytic for the bacterium Escherichia coli.
| Purpose | To determine the titer of a coliphage by the plaque assay method |
| Materials | Overnight broth culture of Escherichia coli strain B Coliphage T2 (1:100 suspension in broth) Test tubes containing 4.5 ml tryptic soy broth Test tubes containing 2.5 ml soft tryptic soy agar (0.7%) Plates of tryptic soy agar at room temperature Sterile 1.0- and 5.0-ml pipettes Pipette bulb or other pipetting device |
Procedures
- Melt the tubes of soft agar and place them in a 50°C water bath.
- Set up a rack with 8 tubes containing 4.5 ml of tryptic soy broth. Label one each of the tubes as follows: 10-3, 10-4, 10-5, 10-6, 10-7, 10-8, 10-9, and “Control.”
- Label 8 soft agar tubes and 8 tryptic soy agar plates in sequence as above beginning with 10-4 and ending with 10-10 and a control (refer to fig. 30.2).
- With a 1.0-ml pipette, remove 0.5 ml of the 1:100 coliphage suspension and place it in the tube of tryptic soy broth labeled 10-3. Discard the pipette.
- With a new pipette, mix the suspension in the tube labeled 10-3 by pipetting up and down several times (use a bulb or other pipetting device). Remove 0.5 ml from this tube and transfer it to the tube of broth labeled 10-4. Discard the pipette.
- Repeat step 5, transferring coliphage from the tube labeled 10-4 to the tube labeled 10-8 and so on until 0.5 ml of phage has been transferred from the tube labeled 10-8 to the tube labeled 10-9.
- With a new pipette, mix the contents of the tube labeled 10-9 and then discard 0.5 ml from this tube into a container of disinfectant. Do not add any coliphage to the tube labeled control.
- Using a 5.0-ml pipette, transfer 0.5 ml of the E. coli overnight culture to each of the melted tubes of soft agar including the control tube. After you add the bacterial inoculum, return each tube to the 507deg;C water bath but move on quickly to the next steps.
- The next procedure will be easier to perform if you work with a partner. Transfer 0.1 ml of the tryptic soy broth from the tube labeled “Control” (in the series of coliphage dilutions) to the tube of soft agar labeled “Control.”Without setting down the pipette or touching it to any surface, hand the tube to your partner. Your partner should mix the contents by rolling the tube between his/her palms and then layering the soft agar over the surface of the tryptic soy agar plate labeled “Control.”The plate should then be rotated and tilted so that the soft agar is spread evenly across the surface of the firmer
- Using the same 1.0-ml pipette as in step 9, remove 0.1 ml of the coliphage suspension from the tryptic soy broth tube
labeled 10-9 and transfer it to the tube of soft agar labeled 10-10. Note that the labels on the broth and soft agar tubes are
not the same (but both are correct). Hand the tube to your partner who will layer the contents carefully over the agar plate
labeled 10-10.
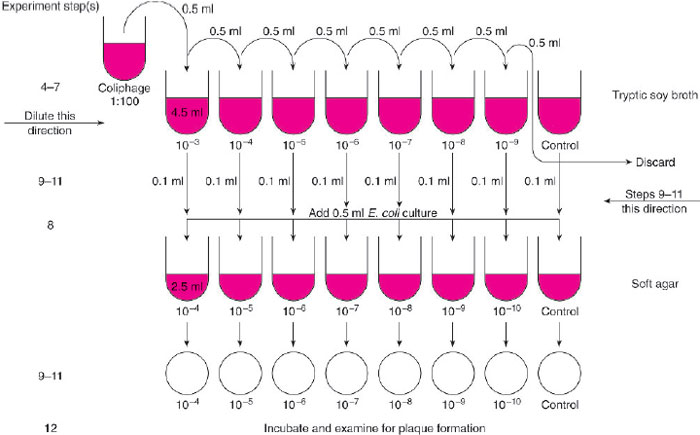
Figure 30.2 Flow diagram for Experiment 30.1.
- Continue to repeat step 10, transferring 0.1 ml from each coliphage dilution to the soft agar tube labeled with the next higher dilution, until you end with the 10-3 dilution in the soft agar tube labeled 10-4. You can use the same pipette throughout unless you think you have contaminated it by touching it to a surface. In each instance, your partner should layer the contents of the tube of soft agar over the corresponding plate of tryptic soy agar.
- After all plates have hardened, incubate them at 37°C until the next session.
- Examine the plates for evidence of the lytic activity of the coliphage on the strain of E. coli. A clear area or “plaque” will appear at each spot where one viral particle attached to and entered one bacterial cell, lysed the cell, and invaded adjacent bacteria.
- Compare the plates that show plaques with the control plate, which should show an even “lawn” of bacterial growth.
- Choose a plate on which the number of plaques is between 30 and 300 and count them. Calculate the original
concentration (plaque-forming units, or PFU) of the coliphage by using the following formula.
PFU/ml of original suspention = number of plaques × 1/plate dilution - Record your results and compare them with those of other groups.
PFU/ml =




