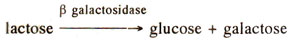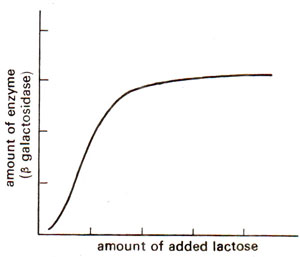
Fig. 35.1. Induction of β galactosidase synthesis by lactose.
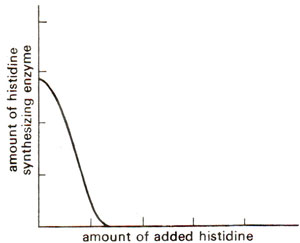
Fig. 35.2. Repression of histidine synthesis enzymes by histidine.
In
E. coli, synthesis of β
galactosidase, an enzyme meant for hydrolysis of lactose into glucose and galactose, has been studied in considerable detail.
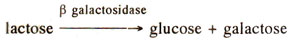
If β galactosides (e.g. lactose) are not supplied to
E. coli cells, the presence of β galactosidase is hardly detectable, but as soon as lactose is added, production of enzyme β galactosidase increases (Fig. 35.1) as much as 10,000 times. The enzyme quantity again falls down as quickly as the substance (i.e. lactose) is removed. Such enzymes, whose synthesis can be induced by adding the substrate are known as
inducible enzymes and the genetic systems responsible for the synthesis of such an enzyme are known as
inducible systems.

Fig. 35.1. Induction of β galactosidase synthesis by lactose.
In some other cases the situation is reverse. For instance, when no amino acids are supplied from outside,
E. coli cells can synthesize all the enzymes needed for synthesis of different amino acids. However, if a particular amino acid like histidine is added, production of histidine synthesizing enzymes will fall down (Fig. 35.2). In such a system the addition of end product of a biosynthetic pathway will check synthesis of the enzymes needed for its biosynthesis. Such enzymes whose synthesis can be checked by adding end product are known as
repressible enzymes and these genetic systems would be known as
repressible systems.

Fig. 35.2. Repression of histidine synthesis enzymes by histidine.