Root diseases
Content
Club root (Plasmodiophora brassicae) This fungus is classified into a quite separate group of fungi, the Plasmodiophorales. Damage: It causes serious damage to most members of the Cruciferae family, which includes cabbage, cauliflowers, Brussels sprouts, stocks and Alyssum. Infected plants show signs of wilting and yellowing of older leaves, and often severe stunting. On examination, the roots appear stubby and swollen (see Figure 15.12), and may show a wet rot.
The disease is favoured by high soil moisture, high soil temperatures and acid soils. Although this fungus does not spread much in undisturbed soils it can be easily carried on infected plants, or on tools and wheels of machinery. In peat soil-growing areas, high winds may carry the disease a considerable distance. Control: Several preventative control measures may be used by the amateur gardener and professional grower. Rotation greatly helps by keeping cruciferous crops away from high spore levels in the soil. Liming of soil greatly inhibits spore activity (see soil fertility). Recently released cultivars of late-summer cabbage are claimed to have strong resistance to club root. Autumn-sown plants establish in soil temperatures unfavourable to the disease and are normally less infected. Compost made from infected brassica plants should be avoided. The professional grower using transplants can prepare a seedbed previously sterilized with a granular product containing dazomet, which would ensure healthy transplants.
Damping off (Pythium and Phytophthora species) These two fungi belong to the Zygomycota. Damage: These two similar genera of fungi cause considerable losses to the delicate seedling stage. The infection may occur below the soil surface, but most commonly the emerging seedling plumule is infected at the soil surface, causing it to topple (see Figure 15.13). Occasionally the roots of mature plants, e.g. cucumbers, are infected, turn brown and soggy, and the plants die. Rose plants often have high levels of Pythium around their roots as they age. Although the mature plant is not seriously affected, it is a common experience that on removal of the plant and replacement with a young rose plant, there is a quite rapid decline in its vigour, called rose-sickness. Life cycle and spread: Both Pythium and Phytophthora occur naturally in soils as saprophytes, but under damp conditions they produce the asexual spores that cause infection. These spores are spread by water. Sexual spores (oospores) are produced in infected roots (mostly in autumn) and may survive several months of dry or cold soil conditions. Control: Prevention control is best achieved (both for the amateur gardener and the professional grower) against these diseases by providing a disease-free growing medium. This may be produced by using fresh compost, by partial sterilization of soil with heat, or (for the professional grower) by a sterilant such as dazomet. Seed producers often coat crop seed with a protective seed dressing (see also) such as thiram to prevent early infection. Water tanks with open tops, harbouring rotting leaves, are a common source of infected water and should be cleaned out regularly. Sand and capillary matting on benches in greenhouses should be regularly washed with hot water. The use of door mats soaked in a sterilant such as dilute formalin may prevent foot spread of the organisms from one greenhouse to another. Waterlogged soils should be avoided, as these fungi increase most rapidly under these conditions. The amateur gardener may use a copper formulation (known as Cheshunt mixture) as a drench to slow down the increase of damping off. Professional growers use a product containing etridiazole, which may be mixed in with composts, or drenched on to seed trays, pots or border soil growing young plants.
Conifer root rot (Phytophthora cinnamomi) This fungus belongs to the Zygomycota group of fungi. Damage: This soil-inhabiting fungus is most commonly a problem in nursery stock production nurseries. It causes the foliage of plants to turn grey-green, then brown and eventually to die off completely (see Figure 15.14). Sliced roots show a chestnut brown rot, with a clear line between infected and non-infected tissues. Two hundred plant species, including Chaemaecyparis, Erica and Rhododendron species may be badly attacked. Life cycle and spread: The disease is commonly introduced on infected stock plants or contaminated footwear. It multiplies most rapidly under wet conditions, within a temperature range of 20°C and 30°C, infecting the root tissues and producing numerous asexual spores, which may be spread by water currents to adjacent plants. Sexual oospores produced further inside the root are released on decay and allow the fungus to survive in the soil for several months without a host. Control: Preventative control (see hygienic growing) is important. Reliable stock plants should be used. Water supply should be checked to avoid contamination. The stock plant area should be elevated slightly higher than the production area to prevent infection by drainage water. Rooting trays, compost and equipment, e.g. knives and spades, should be sterilized (e.g. with formalin) before use. Placing container plants on gravel reduces infection through the base of the pot. The chemical, etridiazole, incorporated in compost protects the roots, but does not kill the fungus. Some species, such as Juniperus horizontalis, have some tolerance to this disease. Honey fungus (Armillaria mellea) This fungus belongs to the Basidiomycota group of fungi.
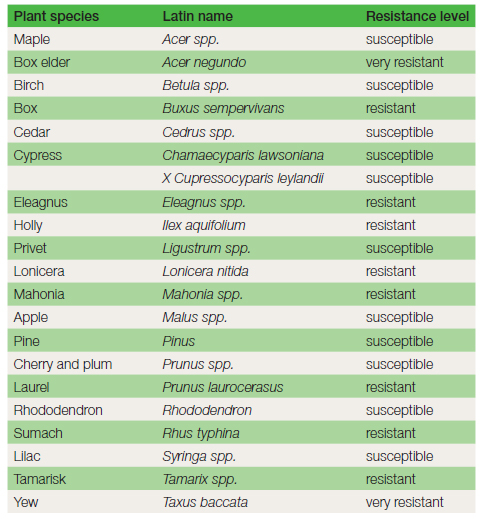
Damage: This fungus primarily attacks trees and shrubs, e.g. apple, lilac and privet. In spring the foliage wilts and turns yellow. Death of the plant may take a few weeks or several years in large trees. Confirming symptoms are the white mycelium, rhizomorphs and toadstools mentioned below (see Figure 15.15). Life cycle and spread: The infection process involves rhizomorphs (sometimes referred to as ‘bootlaces’), which radiate out underground from infected trees or stumps for a distance of 7 m, to a depth of 0.7 m. The infected stump may remain a serious source of infection for twenty years or more. The rhizomorphs are the only means of spread for this disease. The nutrients they are able to conduct provide the considerable energy required for the infection of the tough, woody roots. Mycelium, moves up the stem beneath the bark to a height of several metres and is visible (when the bark is pulled away) as white sheets, smelling of mushrooms. In autumn, clumps of light-brown toadstools may be produced, often at the base of the stem. The millions of spores produced by the toadstools are not considered to be important in the infection process. Honey fungus often establishes itself in newly planted trees and shrubs that have been planted too deeply. Deep planting produces less vigorous plants that are more vulnerable to infection. Vigour is reduced because feeding roots which ideally should be growing near the surface of the soil have been located in the subsoil. Control is difficult. Some genera of plants are less likely to be infected (see Table 15.1). Removal of the disease source, the infected stump, is strongly recommended. In large stumps which are hard to remove a surrounding trench is sometimes dug to a depth of 0.7 m to prevent the progress of rhizomorphs. Loosening soil with a fork and then applying a sterilant, e.g. formalin in a diluted state, may be applied in situations where there are no crops.
Fusarium patch on turf (now called Microdochium nivale) This belongs to the Deuteromycota group of fungi.
Life cycle and spread: Infection of the leaves by spores and hyphae occurs most seriously between 0°C and 8°C, conditions that are found under a layer of snow (hence its other name, snow mould). However, conditions of high humidity at temperatures up to 18°C may result in typical patch symptoms. Spread is by means of water-borne asexual spores under conditions such as autumn dew with no wind. The fungus can survive in frosty or dry summer conditions as dormant mycelium in dead leaf matter or newly infected leaves. Control: Preventative control measures are important. Avoid high soil nitrogen levels in autumn, as this promotes lush, susceptible growth in autumn and winter. Avoid thatchy growth of the turf, as this encourages high humidity and thus favours the disease organism. The groundsman can drench preventative fungicide such as iprodione in autumn to slow down infection of the fungus. Summerapplied systemic fungicide such as thiophanate methyl is able during the actively growing period of the year to move within the plants and achieve curative control. Vascular wilt diseases (Fusarium oxysporum and Verticillium dahliae) These fungi belong to the Deuteromycota group of fungi.
Life cycle and spread: Both organisms may live as saprophytes in the soil. Fusarium survives unfavourable conditions as thick-walled asexual spores, while Verticillium forms small sclerotia. Infection by both genera occurs through young roots or after nematode attack in older roots. The fungal hyphae enter the root xylem tissue and then move up the stem, sometimes reaching the flowers and seeds. The diseases are spread by water-borne asexual spores. The two fungi have different temperature preferences. Verticillium more commonly attacks in springtime, having an optimum infection temperature of 20°C, while Fusarium is more common in summer, with an optimum temperature of 28°C. Control: Control is often necessary in greenhouse crops. Infected crop residues should be carefully removed from the soil at the end of the growing season. The amateur gardener or professional grower may choose to use peat bags instead of soil. The professional may use partial soil sterilization by steam, or a chemical sterilant such as metam-sodium: In unsterilized soils, professional growers may use resistant rootstocks, e.g. in tomatoes, which are grafted onto scions of commercial cultivars. Rotation may be employed against a Fusarium oxysporum attack, as different forms attack different crops. Careful removal of infected and surrounding plants, e.g. in carnations, may slow down the progress of the diseases, especially if the soil area is drenched with a systemic chemical such as carbendazin, which reduces the infection in adjacent plants. |
||||||||||||||||||||||||||||||||||||||