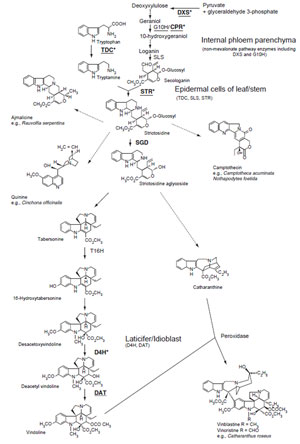
Terpenoid Indole Alkaloid Biosynthesis
Terpenoid indole alkaloids consist of about 3000 compounds, including the antineoplastic vinblastine fromMadagascar periwinkle (Catharanthus roseus), camptothecin from Camptotheca acuminata, and the antimalarial quinine from Cinchona spp. The central intermediate in the biosynthesis of terpenoid indole alkaloids is strictosidine, which is produced from tryptamine and the iridoid glucoside secologanin by STR (Fig. 11.3). Tryptamine is produced by tryptophan decarboxylase (TDC). While a single gene in C. roseus responds in both developmental and inducible expression (De Luca et al., 1989; Goddijn et al., 1992), two genes in C. acuminata showed different expression profiles, suggesting that one is involved in developmentally controlled camptothecin production in shoot apex and bark, while the other is involved in an inducible defense mechanism (Lopez-Meyer and Nessler, 1997).Two steps in secologanin biosynthesis are catalyzed by P450-dependent enzymes: geraniol 10-hydroxylase (G10H) converts geraniol to 10-hydroxygeraniol (Collu et al., 2001), while secologanin synthase (CYP72A1) converts loganin to secologanin and showsepidermis-specific expression in immature leaves of C. roseus (Irmler et al., 2000). The supply of terpenoid precursors should be rate-limiting in terpenoid indole alkaloid biosynthesis. The addition of secologanin or loganin to C. roseus cell culture increases alkaloid accumulation (Whitmer et al., 1998), and the level of G10Hactivity is also positively correlated with the accumulation of alkaloids (Facchini, 2001).However, secologanin is inefficiently used in strictosidine synthesis when added to the medium since exogenous secologanin appears to be compartmentalized differently from endogenous secologanin. This result also suggests that the proper subcellular localization of biosynthetic enzymes and substrates is important for the efficient biosynthesis of metabolites. These isoprenoid precursors are also known to be derived from a nonmevalonate pathway (Contin et al., 1998).
STR is a key enzyme in terpenoid indole alkaloid biosynthesis and cDNAs have been isolated from Rauvolfia serpentina (Kutchan et al., 1988) and C. roseus (Mcknight et al., 1990). STR is one of the most investigated biosynthetic genes in secondary metabolism. Strictosidine is deglucosylated by strictosidine glucosidase (SGD) (Geerlings et al., 2000) and then converted via several unstable intermediates. While there is limited information available on the pathway to catharanthine, vindoline biosynthesis has been relatively well characterized, although the production of vindoline in cultured cells is limited.
The first of six steps in the conversion of tabersonine to vindoline consists of hydroxylation at the C-16 position by tabersonine 16-hydroxylase (T16H), a P450-dependent monooxygenase. While several P450 sequences
were amplified by polymerase chain reaction (PCR), the active principle of CYP71D12 was finally identified to be T16H using translationally fused protein expressed in Escherichia coli (Schroeder et al., 1999). Interestingly, while C. roseus has a single copy of the TDC, STR, and cytochrome P450 reductase (CPR) genes, it has at least two T16H genes. The 16-hydroxylation of tabersonine is followed by 16-O-methylation by a cytosolic SAM: 16 hydroxyltabersonine O-methyltransferase (St-Pierre and De Luca, 1995), hydration of the 2,3-double bond by an as yet uncharacterized enzyme, and N-methylation of the indole-ring nitrogen by a thylakoid-associated SAM: 2,3-dihydro-3-hydroxytabersonine-N-methyltransferase.
The penultimate step in vindoline biosynthesis is catalyzed by a cytosolic 2-oxoglutarate-dependent dioxygenase that hydroxylates the C-4 position of desacetoxyvindoline 4-hydroxylase (D4H) (Vazquez-Flota et al., 1997), and the final step is catalyzed by the cytosolic acetylcoenzyme A: deacetylvindoline 4-Oacetyltransferase (DAT) (St-Pierre et al., 1998). The expression of T16H, D4H, and DAT in developing C. roseus seedlings is light regulated. Although D4H and DAT activities are detected exclusively under conditions that result in vindoline biosynthesis, T16H is expressed at low levels in C. roseus cell cultures that do not accumulate vindoline (St-Pierre and De Luca, 1995). The expression of D4H appears to be under complex, multilevel developmental and light regulation.
A series of experiments with leaves of C. roseus (Burlat et al., 2004; St-Pierre et al., 1999) showed that at least three cell types are involved in vindoline biosynthesis. The nonmevalonate pathway genes (DXS, 1-deoxy-D-xylulose 5-phosphate synthase, 1-deoxy-D-xylulose 5-phosphate reductoisomerase, and 2C-methyl-D-erythriotol 2,4-cyclodiphosphate synthase) as well as G10H were found to be expressed in internal phloem parenchyma of the young aerial organs (Burlat et al., 2004). Other early stage enzymes in the biosynthesis of strictosidine, such as TDC, SLS, and STR, were expressed specifically in the upper and lower epidermis of young leaves, stem, and flower buds. Late-stage enzymes in vindoline biosynthesis, such as D4H and DAT, were localized in laticifer and idioblast cells, which showgreater yellowautofluorescence with fewchloroplasts, compared to the surrounding red-autofluorescent mesophyll cells. Light, which is not required for the formation of these cell types, is required for activation of the localized expression of the late stages of vindoline biosynthesis (Vazquez-Flota et al., 2000).
Vindoline biosynthesis is restricted to the aboveground organs, and the pathway beyond tabersonine is not expressed in tissue cultures (Vazquez-Flota et al., 2002), whereas catharanthine accumulates in cultured cells as well as etiolated seedlings. These facts, along with the recovery of vindoline biosynthesis in regenerated shoots, suggest that the biosynthesis of catharanthine and vindoline is differentially regulated and that vindoline biosynthesis is under more rigid tissue-, developmental-, and environmental-specific control than that of catharanthine (St-Pierre et al., 1999). These results raise the possibility that these cell cultures lack the cell types required to accommodate the late stages of vindoline biosynthesis.
Until recently, characterization of terpenoid indole alkaloid biosynthesis has mainly been carried out with C. roseus, but the recent establishment of hairy root cultures of Ophiorrhiza pumila (Rubiaceae) that showed high camptothecin production provided another useful experimental system. Computer-aided atomic reconstruction of metabolism and tracer experiments with [1–13C] glucose indicated that camptothecin is formed by the combined activities of the 2C-methyl-D-erythritol 4-phosphate pathway and the shikimate pathway (Yamazaki et al., 2004).