Bioengineering Strategies for Generating Plants with Modified Sterol Compositions
Plant metabolic engineering the isoprenoid–phytosterol pathway to understand sterol biosynthesis and function has been underway for about 10 years. The enzymes catalyzing the committed step in the isoprenoid and phytosterol pathways are usually the most important control sites, and in plants they are the HMGR and SMT enzymes, respectively (Bach, 1995; Holmberg et al., 2003; Nes, 2000; Volkman, 2005). Phytosterol synthesis is likely controlled by allosteric interactions involving end products of the pathway and nonsteroidal effectors, changes in the amount of the SMT isoforms, compartmentation and interactions between metabolically distinctive organs. The identification and biochemical characterization of a number of Arabidopsis mutant lines (EMS mutants, T-DNA/Transposon insertion lines, transgenic plants) with an altered sterol profile has enabled researchers in defining the distinct functional metabolic units in the pathway. These mutants also point to the essential roles of sterols in regulating plant development and morphogenesis (Benveniste, 2004). As the genes encoding more enzymes of sterol synthesis are identified and manipulated, it is becoming apparent that phytosterol homeostasis, carbon flux, and growth are intimately tied to a variety of cellular functions and signaling pathways; therefore careful metabolic manipulation of the pathways will be required to generate value-added traits. From the biotechnological perspective the engineering of SMT activity has yet to lead to a desired trait that can be commercialized. However, considerable literature exists on the transgenic alteration of SMTs in plants, which indicates agronomically important applications will be forthcoming.Studies on the results of overexpression and underexpression of the SMT isoforms in tobacco, tomato, potato, and Arabidopsis (Table 9.1) have recently confirmed that SMT1 catalyzes the first step in the cycloartenol-sitosterol (Arnqvist et al., 2003; Fonteneau et al., 1977; Holmberg et al., 2002; Schaeffer et al., 2001; Schaller et al., 2001; Sitbon and Jonsson, 2001) and that SMT2 can regulate the levels of 24-methyl sterol to 24-ethyl sterol in the plant (Arnqvist et al., 2003). SMT1 from plants is feedback inhibited by sitosterol but not by either ergosterol or cholesterol (Nes, 2000). Alternatively, ATP serves as an activator of SMT1 (Nes, 2000). Sitosterol inhibits the SMT1 in a competitive manner by decreasing its affinity for substrates without affecting its Vmax. Sitosterol can also inhibit SMT2 activity but with significantly less effectiveness ca. Ki 100 µM versus 300 µM, respectively (Parker and Nes, 1992). Pulse-chase experiments using radioactively labeled intermediates (Bush and Grunwald, 1973; Heupel et al., 1987; Nes, 1997; Nes, W. D. and Nguyen, H. T., unpublished data) and microarray analysis (Ledford et al., 2004) have shown that light stimulates phytosterol synthesis and the expression of SMT. Three strategies have been employed to genetically modify the phytosterol composition in plants. These strategies are designed to either interrupt or enhance carbon flux at the stage of SMT1 or SMT2 activity or to elaborate improved enzymes aimed at reshaping enzyme specificities and mechanisms (Fig. 9.20).
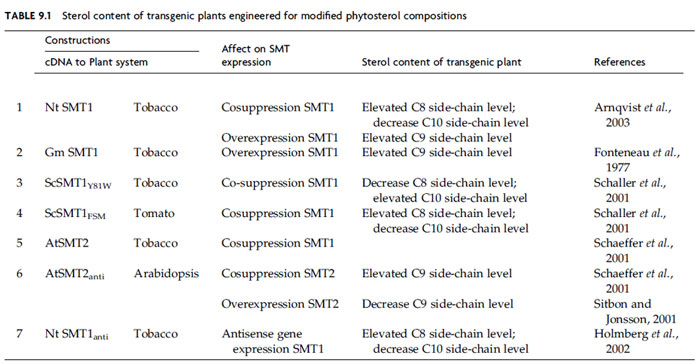 |
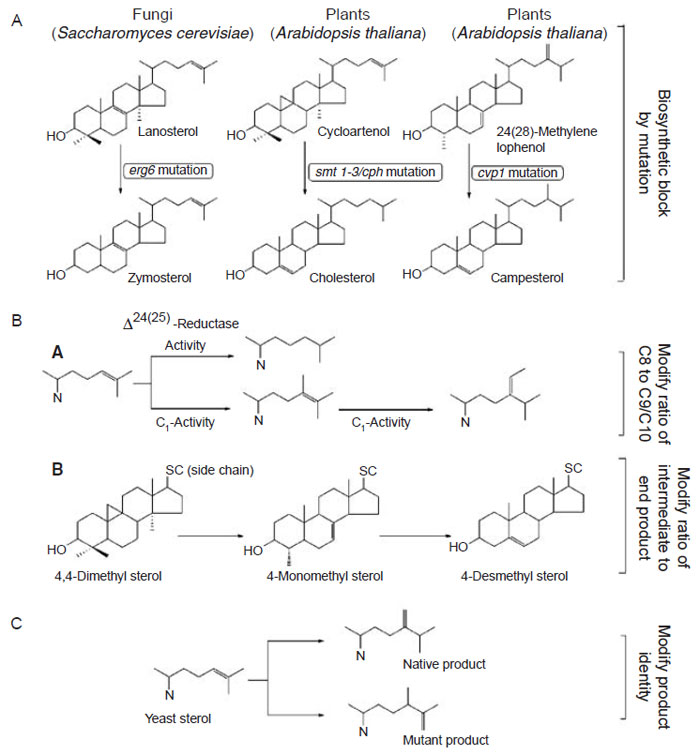 |
| FIGURE 9.20 Different strategies to engineer plants with modified sterol compositions. (A) Engineer change in sterol composition through mutation; (B) engineer change in sterol composition through antisense/cosuppression technology; (C) engineer change through introduction of a foreign SMT that generates novel products. |
 |
| FIGURE 9.21 Comparison of smt1 (Ac-mutagenized plant that generates high levels of cholesterol) and SMT1 (wild-type that generates high levels of sitosterol) plants. (A) Rosette of 2-week plants; (B) rosette of 2-week-old smt1- smt1–3 plant; (C) mature (5-week) SMT1 and smt1–3 plant. Adapted from Diener et al. (2000). (See Page 15 in Color Section.) |
The first approach to modify the phytosterol composition involves mutation of a gene encoding SMT (Diener et al., 2000). Single-enzyme mutation can result in an inability to synthesize the enzyme in active form. Such a defect leads to a block in the metabolic pathway at the point where the enzyme acts and the enzyme’s substrate accumulates. In some cases the functional consequences of the mutation have been investigated (Benveniste, 2004; Diener et al., 2000). The cvp1 Arabidopsis plants defective in SMT2 activity accumulate campesterol, much the same way erg6 yeast mutants defective in SMT1 activity accumulate zymosterol (Fig. 9.20, Panel I) (Ledford et al., 2004). The smt1–3/cph Arabidopsis plants defective in SMT1 activity accumulate cholesterol rather than cycloartenol. The accumulation of cholesterol (nonalkylated sterol) in significant amounts suggests enzymatic reactions which normally do not recognize cycloartenol can process the intermediate to a Δ5-sterol in the transgenic plants. The induced mutations at the stage of either SMT1 or SMT2 activity whereby a 24-desalkyl (C-8-sterol side-chain) sterol accumulates or a 24-methyl (C-9-sterol side-chain) sterol accumulates agrees with the order of intermediates positioned in the kinetically favored pathway of phytosterol biosynthesis. Mutant plants having a modified cholesterol to phytosterol ratio are stunted consistent with the requirement for a fixed phytosterol homeostasis (Fig. 9.21). In related work, inhibitors of sterol biosynthesis administered to cultured plant cells (Nes et al., 1991c) revealed a sequence of reactions in the phytosterol pathway that posits SMT1 as a critical slow step. These transition state analogs possess similar binding properties to either SMT1 or SMT2 (Nes et al., 2003). Therefore, both SMT activities are impaired equally in vivo as well as in vitro. Studies in the design of inhibitors of SMT, activity have not yet progressed to the stage where one compound can inhibit an individual SMT, although mechanism-based inhibitors prepared in our laboratory are tailored with the expectation to be SMT specific (Zhou et al., 2004).
The second approach tomodify the phytosterol composition is to engineer plants with an SMT construct in the sense or antisense direction either of which can lead to cosuppression of native SMT synthesis (Fig. 9.21, Panel B). Phytosterol regulation can favor different branch point enzymes to generate a sterol mixture containing side-chains of varying degrees of C-24 alkylation. If carbon flux in phytosterol synthesis passes along the same set of tracks separated by SMT1 and SMT2, equilibrium considerations will dictate that either slowing or increasing the traffic in one direction by modifyingSMT expression will lead to a change in the ratio of C-8- to C- 9- to C-10-sterol side-chains (Table 9.1). The simplest approach to disturb the steady state concentration of SMT is to engineer either a sense or antisense construct of the native SMT1 to a plant. Transgenic tobacco plants with modified expression of the native SMT1 gene were prepared by introducing sense and antisense expression cassettes of cDNANtSMT1–1 to tobacco plants (Arnqvist et al., 2003;Holmberget al., 2002; Schaeffer et al., 2001; Sitbon and Jonsson, 2001). The resulting plants possessed variations in the cycloartenol proportions and a concomitant effect on the proportion of 24-ethyl sterols. In these plants, the total amount of sterol remained relatively unchanged, consistent with our hypothesis that plants will tend to maintain a sterol homeostasis to the extent possible (Nes, 1990). Arabidopsis plants showing cosuppression of SMT2–1 were characterized by high campesterol levels and depletion of sitosterol (Sitbon and Jonsson, 2001). Pleiotropic effects on development such as reduced growth appear to result from changes in expression of SMT1 and SMT2 (Benveniste, 2004; Lindsey et al., 2003; Schaller, 2004). In a related study, an Arabidopsis frill1 (fri1)mutantwas generated that had amutation inSMT2 and an altered C1/C2-methyl sterol composition. Petalmorphogenesiswas found to be impaired by the change in phytosterol homeostasis (Hase et al., 2005). Overexpression of soybean SMT1 in transgenic potato plants results in a marked reduction of cholesterol and glycoalkaloids (Sitbon and Jonsson, 2001), consistent with the role ofSMT1 to control the level of C-8- to C-9/C-10-sterols.
The reports published thus far on engineering modified sterol pathways in plants revolve around engineering a plant SMT back into plants. An alternative strategy adopted in our laboratory (Nes and Nguyen unpublished data) is to engineer a fungal SMT into plants. We were concerned initially whether plants would either express or otherwise tolerate a fungal SMT. This could be due to either genetic mechanism or due to a failure of the enzyme to catalyze plant substrates which normally are unacceptable to the plant SMT. According to structure–activity tests with the yeast SMT, neither cycloartenol nor 24(28)-methylene lophenol will bind productively (Zhou and Nes, 2003), although zymosterol, the optimal substrate for the yeast SMT1, is a good substrate for SMT2. These findings led to the hypothesis that engineering a yeast SMT to plants will promote the underexpression of the native SMT1 by cosuppression and interrupt carbon flux to SMT2 by providing a foreign SMT that can compete for substrates targeted for SMT catalysis. If substrates normally converted by the plant SMT isoforms were acceptable to the yeast SMT1, then it might be possible to engineer plants with a yeast mutant SMT1 (or some related SMT) capable to generate novel products (Fig. 9.21, Panel C).
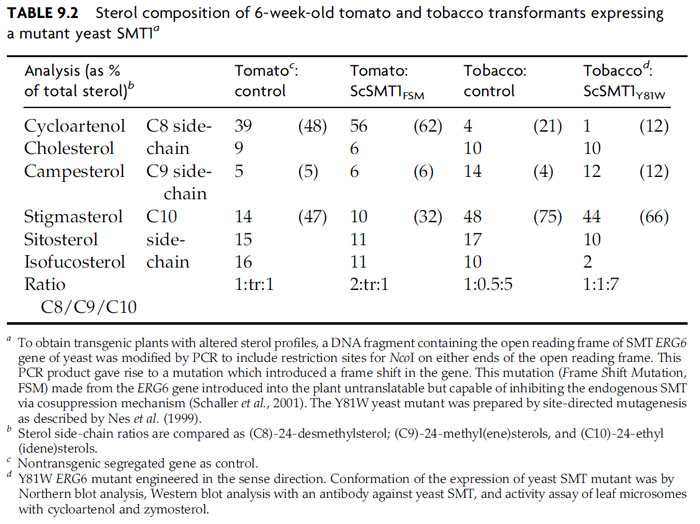
To study our hypothesis further, two different ERG6 SMT constructs were developed. The first ERG6 SMT construct was developed as a consequence of PCR modification which gave rise to a mutation that introduced a frameshift in the gene toward the N-terminus (Nes, unpublished data). This Saccharomyces cerevisiae SMT1 containing a frameshift mutation is referred to as ScSMT1-FSM. A second ERG6 SMT construct was developed from site-directed mutagenesis of an amino acid at position-81 which corresponds to the sterol binding site (Nes et al., 1999). This S. cerevisiae SMT1 containing a tyrosine to phenylalanine mutation at position-81, referred to as ScSMT1-Y81F, was chosen for the engineering studies because it has altered substrate specificity and product sets that make it plant-like. Tomato plants harboring the ScSMT1-FSM transgene contained a high level of cycloartenol and a corresponding decreased level of 24-ethyl sterols (Table 9.2). Tobacco plants harboring the Y81F yeast mutant contained decreased levels of cholesterol and increased levels of 24-ethyl sterols. It is expected that overexpressing a foreign SMT1 in plants will increase the overall SMT1 activity (resulting from the combination of native protein and transgene protein) thereby increasing flux after the formation of 24(28)-methylene cycloartenol, which appears to be the case for either plant or fungal SMTs engineered into plants. The ScSMT1-FSM was not expressed in tomato whereas the Y81F yeast mutant was constitutively expressed, as determined by several techniques including activity assay, Northern blot analysis, and immunochemistry using the yeast SMT antibody (unpublished data). The overexpressed yeast SMT1-Y81F did not appear to compete for endogenous substrates of tobacco since the plant did not make any acceptors suitable for the fungal SMT catalysis.
 |
Because it is generally undesirable to alter the balance of 4,4-dimethyl sterol intermediate to 4-desmethyl sterol end products, except according to the normal developmental program, single-enzyme manipulations designed to either increase the amount of intermediates or change the ratio of C-24-alkylated sterols in the sterol mixture (Fig. 9.21, Panel B), are limited to upregulating HMGR activity and downregulating SMT activity (Bach, 1995; Holmberg et al., 2003; Guo et al., 1995). Generally, changes in the intermediate to end product ratio result in cycloartenol accumulating as sterol ester in lipid droplets whereas the modified ratio of 24-methyl to 24-ethyl Δ5-sterols results in little change in the total cellular sterol thereby maintaining sterol balance in the membrane.
The third approach being developed in our laboratory is to engineer a combination of transgenes in the antisense and sense directions to crop plants. We expect to eliminate the expression of the native SMT1 thereby permitting a foreign SMT to be expressed that possesses an unusual catalytic
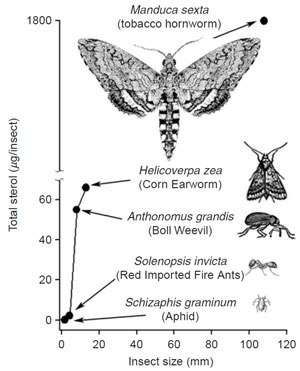 |
| FIGURE 9.23 Correlation of sterol content with insect size. Adapted from Behmer and Nes, 2003. |
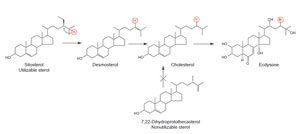
competence (Fig. 9.21, Panel C). This strategy is based on the observation that insects do not synthesize their own sterol and require cholesterol to synthesize ecdysteroid involved in the molting process (Behmer and Nes, 2003). Phytophagous insects convert sitosterol, obtained from the host plant, to cholesterol (Fig. 9.22) (Behmer and Nes, 2003; Nes et al., 1997). Insects, like plants, have different sterol requirements depending on size and with respect to their phylogeny (Fig. 9.23). In the C-24-dealkylation pathway, the hydrogen atom at C-25 migrates to C-24 during the elimination of the 24-ethyl group. Thus, phytosterols with a Δ25(27)-bond will not undergo side-chain metabolism by the insect; therefore these sterols will be nonutilizable nutrients for growth (Svoboda et al., 1995 Nes et al., 1997). In order to develop insect-resistant plants, a strategy we considered is to redesign the yeast SMT1 to affect its catalytic competence in such a way to bind cycloartenol, to remove the affinity for effectors (sitosterol) that might interfere with SMT activity, and to modify the active site to promote channeling to generate Δ25(27)-olefins. It is clear that we can tailor SMTs to produce new substrate affinities and products. The opportunities to generate transgenic plants that appear similar to wild types with modified sterol compositions are limitless and soon the commercial benefits of this evolving bioengineering technology directed at phytosterols will be realized.