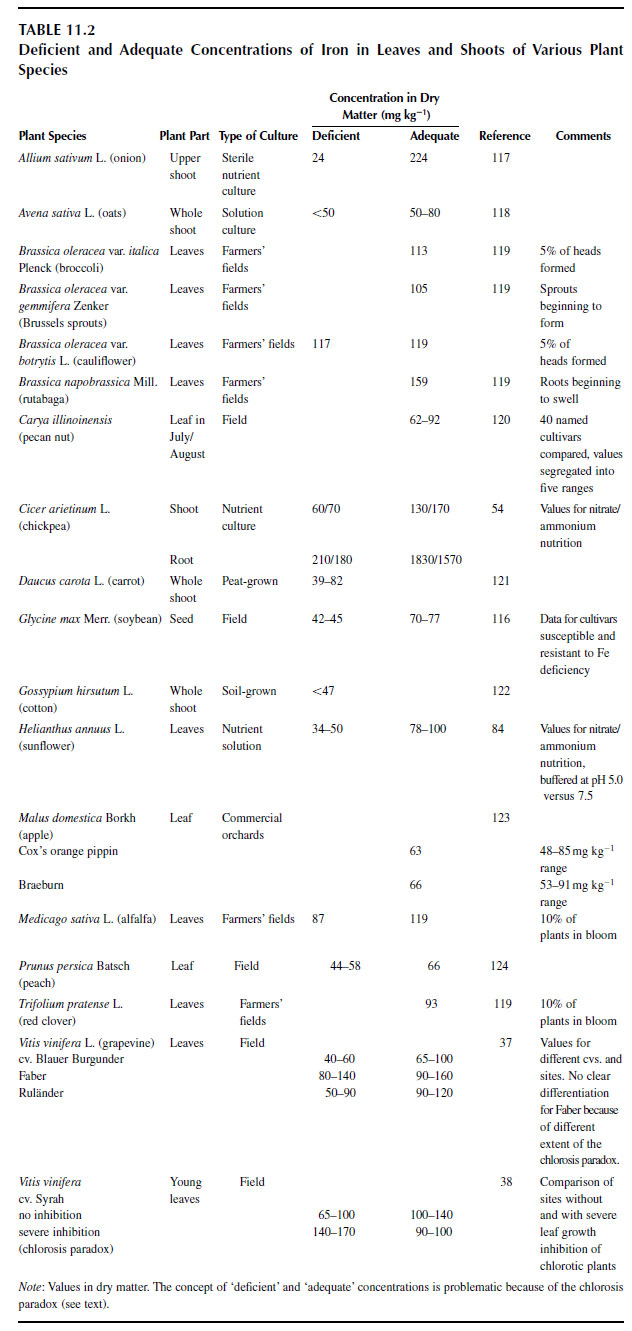
Factors Affecting Plant Uptake
Soil Factors
The major factor affecting acquisition of iron by plants is soil pH, with high pH making iron
less available and giving rise to chlorosis. Along with lime-induced chlorosis, there is a whole range
of factors, including the weather, soil and crop management, and the plant genotypes themselves,
that give rise to chlorosis by impeded uptake of iron (
Table 11.1). In lime-induced chlorosis, it is the soil bicarbonate that is the key cause, largely due to the high pH in the rhizosphere and at the
root uptake site, thereby affecting iron solubility and Fe(III)-chelate reductase activity (
see Section).
One factor that may contribute to rhizosphere pH changes, other than the underlying substrate,
is the nitrogen source. When plants take up nitrate as their predominant nitrogen source, they alkalinize
the rhizosphere and this contributes to iron deficiency stress
(84,93,94). It has been suggested
that nitrate nutrition could actually raise the pH in the leaf apoplast, making iron less available for
transport into leaf cells. However, this assumption was not experimentally confirmed (
see Section).
Chlorosis in plant species with Strategy 1 is made worse by high soil moisture, particularly on
calcareous soils, because of elevated concentrations of bicarbonates. A peach tree that was overirrigated
in an orchard on a calcareous soil developed bicarbonate-induced chlorosis, whereas a tree
that received proper irrigation showed no chlorosis (Figure 11.7). In addition, anaerobiosis may
make root responses to iron deficiency stress more difficult
(13). Organic matter content of the soil
can also be important, partly because of the increased tendency toward waterlogging in organic soils
lowering iron availability, but also because of enhanced microbial activity and the presence of
chelating agents in the organic matter making iron more available
(13). Furthermore, soil organic
matter, and also compaction of soil, could lower root growth and inhibit iron uptake because of generation
of ethylene
(95). Low temperature can make chlorosis more extreme because of the slower
metabolic processes in the roots inhibiting the iron-deficiency responses; very high concentrations
of soil phosphate can be deleterious through the adsorption of phosphates on to iron oxides; high
soil solution osmotic strength appears to lower the effectiveness of iron chelation in Strategy 1
plants; and high concentrations of Cu, Zn, and Mn can induce iron chlorosis through replacement
of iron in soil chelates and phytosiderophores and inhibition of the iron-deficiency responses
(13).
A summary of the interactions between environmental, edaphic and management conditions, and
plant genotype, concerning the onset of chlorosis is shown in Figure 11.8.
 |
| FIGURE 11.7 Two peach (Prunus persica Batsch) trees in an orchard on a calcareous soil with drip irrigation.
Left: over-irrigation by a defect dripper resulting in bicarbonate-induced chlorosis. Right: adequate irrigation,
no chlorosis. |
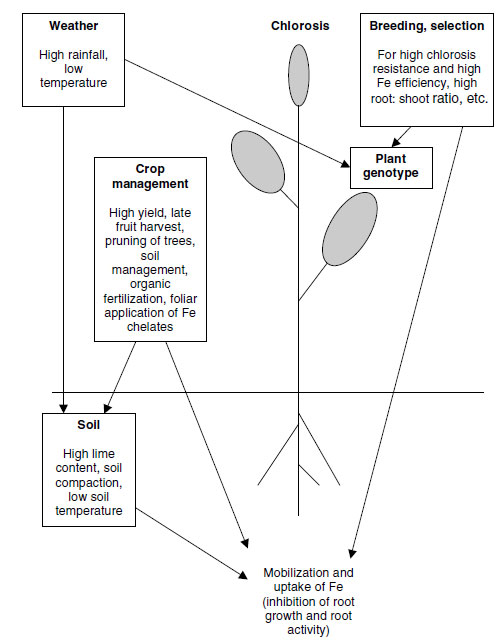 |
| FIGURE 11.8 Causal factors of chlorosis and their interactions responsible for the onset of Fe-deficiency chlorosis in plants. (Redrawn from Kirkby, E.A., R�mheld, V., Micronutrients in Plant Physiology: Functions, Uptake and Mobility. Proceedings No. 543, International Fertiliser Society, Cambridge, U.K., December 9,
2004, pp. 1-54.) |