Isolation and Identification of Streptococci
The genus Streptococcus contains gram-positive cocci that characteristically are arranged in chains (see colorplate 2). A number of species of streptococci are normally found among the normal flora of human skin and mucous membranes, particularly those of the upper respiratory tract. Certain species are more commonly associated with human infectious diseases than others.Many streptococci have fastidious growth requirements including a requirement for blood-enriched media. Most grow well in air but also grow in the absence of oxygen (i.e., they are facultative anaerobes), some prefer reduced oxygen tension and increased CO2 (microaerophilic), and some grow only in the absence of oxygen (anaerobic). The anaerobic streptococci are now placed in the genus Peptostreptococcus. An incubation temperature of 35°C is optimal for growth of most streptococci.
A number of streptococcal species produce substances that destroy red blood cells; that is, they cause lysis of the red cell wall with subsequent release of hemoglobin. Such substances are referred to as hemolysins. The activity of streptococcal hemolysins (also known as streptolysins) can be readily observed when the organisms are growing on a blood agar plate (see colorplate 11). Different streptococci produce different effects on the red blood cells in blood agar. Those that produce incomplete hemolysis and only partial destruction of the cells around colonies are called alpha-hemolytic streptococci. Characteristically, this type of hemolysis is seen as a distinct greening of the agar in the hemolytic zone, and thus this group of streptococci has also been referred to as the viridans group (from the Latin word for green).
Species whose hemolysins cause complete destruction of red cells in the agar zones surrounding their colonies are said to be beta-hemolytic. When growing on blood agar, beta-hemolytic streptococci are small opaque or semitranslucent colonies surrounded by clear zones in an otherwise red opaque medium. One of the two streptococcal hemolysins involved in this reaction is inhibited by oxygen. Its effect is seen best around subsurface colonies or when culture plates are incubated anaerobically. Some strains of staphylococci, Escherichia coli, and other bacteria also may show beta-hemolysis.
Some species of streptococci do not produce hemolysins. Therefore, when their colonies grow on blood agar, no change is seen in the red blood cells around them. These species are referred to as nonhemolytic streptococci, although formerly, they were called gamma streptococci. Colorplate 28 shows the appearance of alpha-, beta-, and nonhemolytic streptococci growing as subsurface colonies.
In the clinical microbiology laboratory, observation of hemolysis is an important first step in differentiating among streptococcal species. Alpha-hemolytic streptococci are members of the normal throat flora and do not need to be identified further when they are isolated from respiratory cultures. In persons with heart valve abnormalities, however, these streptococci may be deposited on the valves, usually at the time of dental work when they have the opportunity to enter and circulate through the bloodstream. The inflammatory process that develops on the valve is referred to as endocarditis and, if untreated, is a lifethreatening infection. In patients with endocarditis, isolation of viridans streptococci from multiple blood cultures is considered diagnostic of endocarditis.
When beta-hemolytic streptococci are found in throat cultures, the laboratory must proceed with further testing to determine the antigenic group. This is done by extracting the carbohydrate antigen from the streptococcal cell wall and reacting it with specific antibodies in a latex agglutination test or enzyme immunoassay. The most important streptococcal group is group A, which is responsible for streptococcal pharyngitis (“strep throat”) and a variety of other serious skin and deep tissue infections (see table 21.1). The species name given to group A streptococci is pyogenes (pus producing, a characteristic of the infection produced). Certain toxins and extracellular products of S. pyogenes are responsible for scarlet fever, rheumatic fever, and a toxic shock syndrome similar to that produced by Staphylococcus aureus.
At one time, group B streptococci (Streptococcus agalactiae) were considered primarily animal pathogens. Now, they are known to colonize the human female vaginal tract. In some colonized pregnant women, the organism causes infection of the endometrium following delivery, and more seriously, may produce sepsis and meningitis in their newborn child. Because of these severe infections, pregnant women are routinely screened for vaginal carriage of the group B Streptococcus a few weeks before term, and treated with antimicrobial agents if they are colonized.
Other beta-hemolytic streptococci are placed in groups C through V, but most do not cause disease. Groups C, F, and G may cause mild pharyngitis but do not have the serious effects that groups A and B do. Streptococcal-like bacteria with group D antigen were at first classified in the genus Streptococcus, but studies have revealed that they differ in many biological respects. Therefore, they have now been placed in their own genus, Enterococcus.
Identification of Streptococci
In smears from patient material, the microbiologist presumptively identifies gram-positive cocci in chains as streptococci (although the Gram-stain reaction and morphology only are reported to the physician). When culture growth is available, the type of hemolysis produced by colonies on blood agar plates leads to the next step(s) in identification. Alpha-hemolytic colonies from respiratory specimens are not identified further because they are considered normal flora. Beta-hemolytic colonies must be identified to determine whether or not they are group A (any specimen type) or group B (genital specimens from pregnant women).
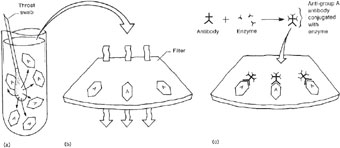
Although serological testing (most commonly by the latex agglutination method, fig. 19.2) is the definitive method for grouping beta-hemolytic streptococci, rapid methods for grouping have become available only recently and they are expensive. Therefore, alternative, presumptive tests are commonly used in the laboratory for identifying groups A and B streptococci. For example, group A streptococci, but not other beta-hemolytic streptococci, are susceptible to low concentrations of the drug bacitracin. By using a bacitracin disk-diffusion assay such as you performed in Experiment 15.1, the susceptibility of suspected strains can be tested (see colorplate 29). Group B streptococci produce a substance called the CAMP factor (see Experiment 21.2 and colorplate 30) that enhances the effect of beta-hemolysins possessed by some strains of Staphylococcus aureus. All other groups of beta-hemolytic streptococci must be identified serologically, but in practice, determining the absence of group A and B strains is usually sufficient for clinical purposes.
In addition to using a rapid latex agglutination test for identifying group A streptococci (fig. 21.1), a rapid enzyme immunoassay test (see figs. 21.2 and 19.4) is available to detect the group A antigen directly from a throat swab, without first growing the organism in culture. This type of test is usually performed in clinics and in physicians’ offices because it is rapid (10 to 30 minutes) and does not require culture expertise. However, for a positive test, a large number of organisms is needed on the swab. When negative results are obtained for patients with clinical evidence of pharyngitis, a throat swab for “strep” culture should always be sent to the clinical microbiology laboratory.
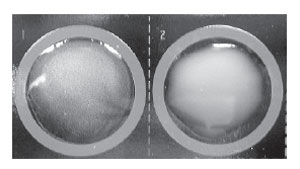 |
| Figure 21.1 A positive latex agglutination reaction for group A streptococci. The left-hand well shows a very fine, granular precipitate. In this well, the group A carbohydrate antigen has combined with latex beads coated with antibody against this specific antigen. The well on the right (group B antigen) remains negative, showing only the milky suspension of nonagglutinated latex particles. This antigen does not react with the anti–group A antibodies on the latex particles. |
|
|||
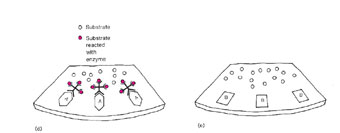
| Figure 21.2 Diagram of a streptococcal enzyme immunoassay (EIA). (a) A throat swab from a patient with streptococcal pharyngitis is placed in a tube with extraction solution, which extracts the group A antigen. (b) The extraction solution is then passed through a filter where the group A antigen attaches to its surface. (c) After a wash step, an antibody against the group A Streptococcus, which is linked to an enzyme, is added to the filter where it attaches to the group A antigen. (d) After another wash, a colorless substrate specific for the enzyme is added and is split to a colored end product when it comes in contact with the antibody-bound enzyme. (e) If an antigen other than group A was present, no antibody would bind. Unbound antibody would be washed away, and no color reaction would be seen. |
Table 21.1 summarizes the characteristics of streptococci and enterococci. In Experiment 21.1, we shall study some simple methods for isolating streptococci from clinical specimens and for presumptive and confirmatory identification of betahemolytic strains as group A. In Experiment 21.2, procedures for differentiating group B strains by the CAMP and serological tests will be followed.
| Purpose | To isolate and identify streptococci in culture |
| Materials | Sheep blood agar plate Simulated throat culture from a 2-year-old child with acute tonsillitis Demonstration blood agar plate showing alpha-hemolytic, beta-hemolytic, and nonhemolytic strains of streptococci Demonstration plate showing response of two strains of beta-hemolytic streptococci to bacitracin disks (A disks) Solution with extracted antigen of beta-hemolytic Streptococcus (prepared by instructor) Latex test kit for serological typing |
Procedures
- Inoculate and streak a blood agar plate with the simulated clinical specimen. Make a few stabs in the agar at the area of heaviest inoculum. Try not to stab to the bottom of the agar medium layer.
- Incubate the plate at 3°C for 24 hours.
- Examine the demonstration plates (but do not open them without supervision).
- Following the manufacturer’s directions, use the typing kit to identify serologically the beta-hemolytic isolate. Mix the antigen extract with a drop of each of the group A and group B latex reagents.
- Observe both suspensions for evidence of agglutination.
- Record your observations under Results (no. 7) (fig. 21.1).
Results
- Describe the “patient’s” throat culture results in the following table, after making Gram stains.
- Describe any differences in intensity of hemolysis around colonies growing on the agar surface and those pushed below the surface where you stabbed into the agar.
- How would you report the culture results to the physician?
- Demonstration plate showing different types of hemolysis: describe your observations of:
- Alpha-hemolytic streptococci
- Beta-hemolytic streptococci
- Nonhemolytic streptococci
- Demonstration plate with A-disks: diagram your observations, indicating the position of the disks, areas of growth, and
type of hemolysis.
- State your interpretation of the bacitracin disk results.
- With which latex reagent did you obtain a positive result? Group
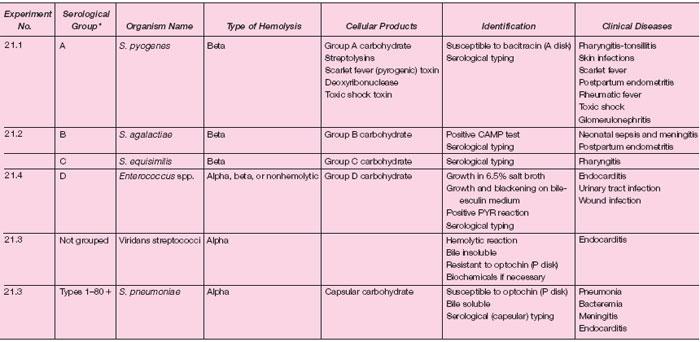 |
| Table 21.1 Classification and Identification of Streptococci, Pneumococci, and Enterococci |