Special Stains
Some bacteria have characteristic surface structures (such as capsules or flagella) and internal components (e.g., endospores) that may have taxonomic value for their identification. When it is necessary to demonstrate whether or not a particular organism possesses a capsule, is flagellated, or forms endospores, special staining techniques must be used.Many bacterial species possess an exterior capsule composed of carbohydrate or glycoprotein. A few pathogenic species, such as Streptococcus pneumoniae (the leading cause of bacterial pneumonia) and Klebsiella pneumoniae (a cause of pneumonia and wound and urinary tract infections) have well-developed capsules that contribute to virulence by preventing phagocytic cells from ingesting and killing the bacteria. Capsules do not retain staining agents, but can be made visible microscopically by the use of a simple, nonspecific negative staining technique. A small drop of India ink or nigrosin is added to a suspension of bacterial cells on a glass slide. These agents do not penetrate the cells (or stain the surrounding capsules), but serve as background stains, which outline the capsules. When the slide is dry, the preparation is stained with safranin, which penetrates and stains the cells. After this treatment, the bacteria appear pink and their capsules stand out sharply as clear, unstained zones against the dark background of India ink or nigrosin.
In the animal body, carbohydrate or protein capsular components are recognized as foreign substances (referred to as antigens). In response to the presence of capsular antigens, antibodies are produced that react with (bind to) the capsule. The binding of antibodies to antigen (called an antigen-antibody reaction) greatly enhances phagocytosis. Many variations in the chemical structure of capsular antigens exist, even within a single bacterial species, and each variation stimulates the production of antibodies specific for that capsular type. For example, more than 80 capsular types of S. pneumoniae have been identified.
Serological tests, which make use of these specific antigen-antibody reactions, are often used for rapid and accurate identification of bacteria present in clinical specimens. Encapsulated bacteria may be identified serologically by one such test, called the quellung reaction. In this test, unknown bacteria from the clinical specimen are placed on a slide and antiserum (serum containing known antibodies) is added. When the preparation is viewed under the microscope, if an antigen-antibody reaction has occurred between antibodies in the test serum and capsular antigens on the bacterial cells (a positive test), the capsules become much more distinct and appear to swell (see colorplate 10).
Bacterial flagella are tiny hairlike organelles of locomotion. Originating in the cytoplasm beneath the cell wall, they extend beyond the cell, usually equaling or exceeding it in length. Their fine protein structure requires special staining techniques for demonstrating them with the light microscope. Since not all bacteria possess flagella, their presence, numbers, and pattern or arrangement on the cell may provide clues to identification of species (fig. 7.1). For example, Vibrio cholerae and some species of Pseudomonas have a single polar flagellum at one end of the cell (they are said to be monotrichous), some spirillae display bipolar tufts of flagella (the arrangement is called lophotrichous), while many Proteus species have multiple flagella surrounding their cells (in a peritrichous pattern). Some flagellar stains employ rosaniline dyes and a mordant, applied to a bacterial suspension fixed in formalin and spread across a glass slide. The formalin links to, or “fixes,” the flagellar and other surface protein of the cells. The dye and mordant then precipitate around these “fixed” surfaces, enlarging their diameters, and making flagella visible when viewed under the microscope. Inanother method, a ferric-tannate mordant and a silver nitrate solution are applied to a bacterial suspension. The resulting dark precipitate that forms on the bacteria and their flagella allows them to be easily visualized under the microscope. This silver-plating technique is also used to stain the very slender spirochetes.
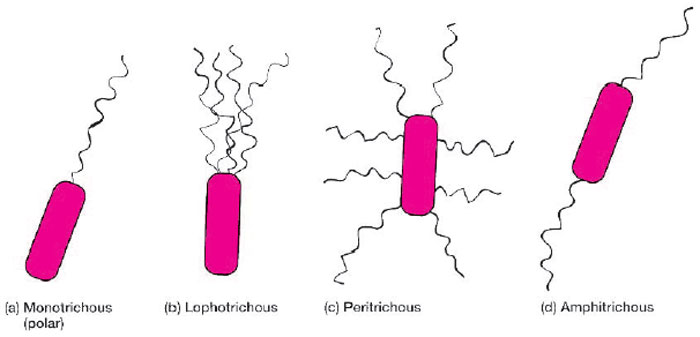 |
| Figure 7.1 Arrangements of bacterial flagella. |




