Quinoline and Acridine Alkaloids
Alkaloids derived from anthranilic acid undoubtedly occur in greatest abundance in plants from the family the Rutaceae. Particularly well represented are alkaloids based on quinoline and acridine skeletons (Figure 106). Some quinoline alkaloids such as quinine and camptothecin have been established to arise by fundamental rearrangement of indole systems and have their origins in tryptophan. A more direct route to the quinoline ring system is by the combination of anthranilic acid and acetate/malonate, and an extension of this process also accounts for the origins of the acridine ring system (Figure 3.47, page 81). Thus, anthraniloyl-CoA (Figure 108) can act as a starter unit for chain extension via one molecule of malonyl-CoA, and amide formation generates the heterocyclic system, which will adopt the more stable 4-hydroxy-2-quinolone form (Figure 108). Position 3 is highly nucleophilic and susceptible to alkylation, especially via dimethylallyl diphosphate in the case of these alkaloids.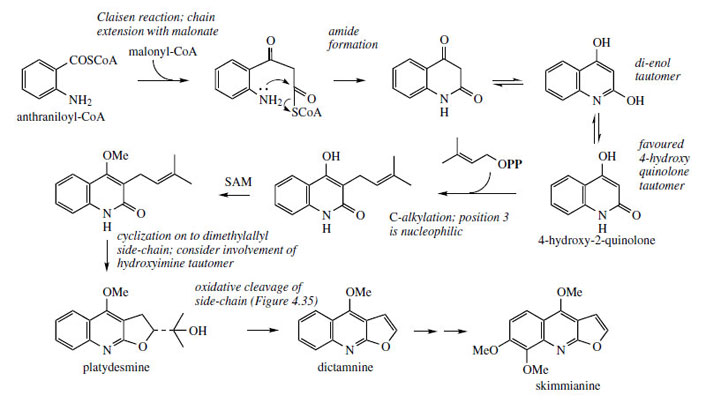 |
| Figure 108 |
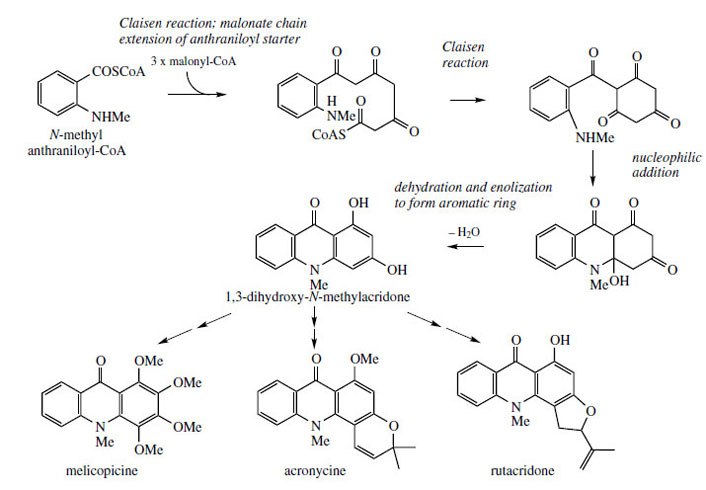 |
| Figure 109 |
This allows formation of additional six- and five-membered oxygen heterocyclic rings, as seen with other systems, e.g. coumarins, isoflavonoids, etc. By an analogous series of reactions, the dimethylallyl derivative can act as a precursor of furoquinoline alkaloids such as dictamnine and skimmianine(Figure 108). These alkaloids are found in both Dictamnus albus and Skimmia japonica (Rutaceae). To simplify the mechanistic interpretation of these reactions, it is more convenient to consider the di-enol form of the quinolone system.
Should chain extension of anthranilyl-CoA (as the N-methyl derivative) incorporate three acetate/ malonate units, a polyketide would result (Figure 109). The acridine skeleton is then produced by sequential Claisen reaction and C–N linkage< by an addition reaction, dehydration, and enolization leading to the stable aromatic tautomer. Again, the acetate-derived ring, with its alternate oxygenation, is susceptible to electrophilic attack, and this can lead to alkylation (with dimethylallyl diphosphate) or further hydroxylation. Alkaloids melicopicine from Melicope fareana, acronycinefrom Acronychia baueri, and rutacridone from Ruta graveolens (Rutaceae) typify some of the structural variety that may then ensue (Figure 109).




