Malaria, leishmaniasis and trypanosomiasis
MalariaMalaria is caused Plasmodium falciparum, P. vivax, P. ovale or P. malariae. More than 1 million children under the age of 5 years die each year in Africa alone. In the UK there are more than 2000 reported cases and up to 10 deaths every year. Immigrants returning to their home country are at high risk as they have lost their natural immunity and may not take prophylaxis.
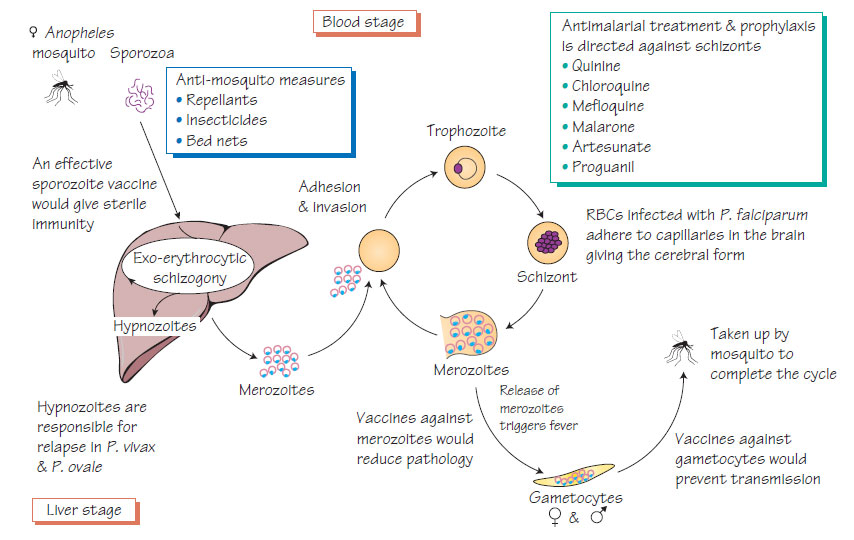
Malaria is transmitted by the bite of a female anopheles mosquito that injects sporozoa. The parasites develop in hepatocytes then invade red blood cells and multiply rapidly. They provoke the release of cytokines, which are responsible for many of the signs and symptoms of malaria. Infected red blood cells develop knoblike projections that make them adhere to the capillary wall. This may occur in the brain, causing cerebral malaria. The life cycle is completed when the sexual stages (gametocytes) are taken up by another mosquito and develop in the mosquito gut into sporozoites, which migrate to the insect salivary glands ready for another bite. P. vivax and P. ovale develop dormant stages (hypnozoites) that cause relapses.
- Fever or 'flu-like symptoms, although there may be no 'typical' malaria symptoms.
- Residence or travel history.
- Rapid progression is possible in P. falciparum infection in the young or travellers.
- Complications of P. falciparum may include cerebral malaria, circulatory shock, acute haemolysis and renal failure, hepatitis and pulmonary oedema.
- Other species are associated with milder, more chronic disease.
Diagnosis
- Urgent blood film examination - usually three.
- Antigen detection tests are useful where laboratories have less experience.
- Nucleic acid amplification tests (NAATs) are used to detect drug resistance.
- Artesunate combination therapy or quinine is recommended for management of P. falciparum malaria.
- Intensive care support may be required.
- Chloroquine and primaquine are used for other malaria species.
Prevention and control
- Insecticide-treated bed-nets.
- Insecticide room spraying.
- Covering of exposed skin between dusk and dawn.
- Prophylaxis should be taken according to expert, up-to-date advice.
- Despite extensive research no vaccine is yet available.
Leishmaniasis
This is a protozoan disease transmitted by sandflies, which inject infective promastigotes that adapt to survival in cells of the reticuloendothelial system as amastigotes.
Two disease forms exist: visceral disease (Leishmania donovani, L. infantum or L. chagasi) and cutaneous disease (L. major, L. tropica and L. aethiopica in the Old World and L. braziliensis and L. mexicana in the Americas).
- Visceral disease: Fever and wasting are important symptoms. The bone marrow, liver and spleen are infiltrated by parasites so the patient becomes anaemic, leucopenic and thrombocytopenic. Reactive hypergammaglobulinaemia makes patients susceptible to secondary bacterial infections. Untreated patients will deteriorate and die within 2 years.
- Cutaneous disease: Chronic, circular skin lesions occur at the site of the bite, with satellite lesions. Some infections with L. braziliensis may cause destruction of structures around the mouth and nose (espundia).
- Microscopy of skin biopsy, bone marrow sample, blood sample or splenic aspirate and culture.
- NAAT is used for primary diagnosis and speciation.
- Serological diagnosis by direct agglutination and dipstick tests for field use.
- Visceral and cutaneous leishmaniasis can be treated with parenteral liposomal amphotericin B. Alternatives include antimony compounds, paromomycin and oral miltefosine.
Trypanosomiasis
African trypanosomiasis
African trypanosomiasis, caused by Trypanosoma brucei gambiense and T. brucei rhodesiense, is transmitted by the tsetse fly. Humans are the only host of T. brucei gambiense, but antelope or cattle act as the reservoir for T. brucei rhodesiense. Parasites in the blood are inhibited by immune responses, but antigenic variation allows the organisms to multiply again. Generalized lymphadenopathy may be present and the skin may appear oedematous. The patient exhibits a hypergammaglobulinaemia and is susceptible to secondary bacterial infection. When parasites invade the brain they cause chronic, progressive encephalitis and the patient lapses into coma. Death is often the result of secondary bacterial pneumonia.
Parasites are demonstrated in blood, CSF or lymph-node aspirate. Serological tests are available. Lumbar puncture should only be performed after circulating parasites have been eliminated with primary treatment. Primary treatment T. brucei gambiense is treated with pentamidine; T. brucei rhodesiense with suramin. Secondary treatment of cerebral infection T. brucei gambiense is treated with melarsoprol or eflornithine +/- nifurtimox; T. brucei rhodesiense with melarsoprol.
South American trypanosomiasis
Trypanosoma cruzi, which causes Chagas disease, is transmitted by the bite of reduviid bugs. There are three phases of the disease: acute infection characterized by cutaneous oedema, intermittent fever, shock and a significant mortality in children; latent infection; and late manifestations, such as achalasia, megacolon, cardiac dysrhythmias, cardiomyopathy and neuropathy.
Parasites are demonstrated by microscopy, or culture in artificial media or laboratory bugs (xenodiagnosis). Serological tests are available.
Treatment
Nifurtimox and benznidazole are useful in the acute phase but efficacy declines as infection progresses. Treatment of complications is mainly palliative (e.g. cardiac pacemakers for heart block secondary to cardiomyopathy, surgery for megacolon).