Glycolysis and Fermentation
Carbohydrates are a major source of energy for organisms. The major pathway by which carbohydrates are degraded is called glycolysis. Starch, glycogen, and other carbohydrates are converted to the sugar glucose by pathways. In glycolysis, glucose, a sixcarbon sugar, is oxidized and cleaved by enzymes in the cytoplasm of cells to form two molecules of pyruvate, a three-carbon compound (see Figs. 3 and 4). The overall reaction is exergonic and some of the energy released is conserved by coupling the synthesis of ATP to glycolysis.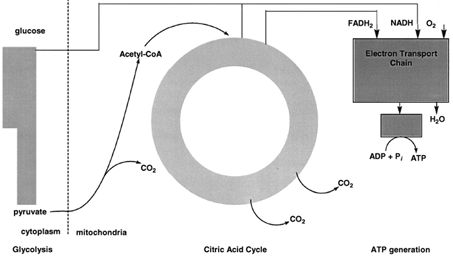 |
| Figure 3 Schematic outline of carbohydrate metabolism. Glucose is oxidized to two molecules of pyruvate by glycolysis in the cytoplasm. In mitochondria, pyruvate is oxidized by molecular oxygen to CO2 and water. The synthesis of ATP is coupled to pyruvate oxidation. |
Before it may be metabolized, glucose must first be phosphorylated on the hydroxyl residue at position 6. Under intracellular conditions, the direct phosphorylation of glucose by Pi is an unfavorable reaction, characterized by a ΔG0' 0 of about 4 kcal/mol, at pH 7.0 and 25°C. (Note that the biochemist’s standard state differs from that as usually defined in that the activity of the hydrogen ion is taken as 10−7 M, or pH 7.0, rather than 1 M, or pH 0.0. pH 7.0 is much closer to the pH in most cells.) This problem is neatly solved in cells by using ATP, rather than Pi, as the phosphoryl donor:
| Glucose + ATP ↔ Glucose 6-phosphate + ADP. |
The ΔG0' for this reaction, which is catalyzed by the enzyme hexokinase, is approximately −4 kcal/mol. Thus the phosphorylation of glucose by ATP is an energetically favorable reaction and is one example of how the chemical energy of ATP may be used to drive otherwise unfavorable reactions.
Glucose 6-phosphate is then isomerized to form fructose 6-phosphate, which in turn is phosphorylated by ATP at the 1-position to form fructose 1,6-bisphosphate. It seems odd that a metabolic pathway invests 2 mol of ATP in the initial steps of the pathway when ATP is an important product of the pathway. However, this investment pays off in later steps.
Fructose 1,6-bisphosphate is cleaved to form two triose phosphates that are readily interconvertible. Note that the oxidation–reduction state of the triose phosphates is the same as that of glucose 6-phosphate and the fructose phosphates. All molecules are phosphorylated sugars. In the next step of glycolysis, glyceraldehyde 3-phosphate is oxidized and phosphorylated to form a sugar acid that contains a phosphoryl group at positions 1 and 3. The oxidizing agent, nicotinamide adenine dinucleotide (NAD+), is a weak oxidant (E0´, at pH 7.0 of −340 mV). The oxidation of the aldehyde group of glyceraldehyde 3-phosphate to a carboxylate is a favorable reaction that drives both the oxidation and the phosphorylation. This is the only oxidation–reduction reaction in glycolysis.
The hydrolysis of acyl phosphates, such as that of position 1 of 1,3-bisphosphoglycerate, is characterized by strongly negative ΔG0' values. That for 1,3-bisphosphoglycerate is approximately −10 kcal/mol, which is significantly more negative than the ΔG0' for the hydrolysis of ATP to ADP and Pi. Thus, the transfer of the acyl phosphate from 1,3-bisphosphoglycerate to ADP to form 3-phosphoglycerate and ATP is a spontaneous reaction. Since two sugar acid bisphosphates are formed per glucose metabolized, the two ATP invested in the beginning of the pathway have been recovered.
In the next steps of glycolysis, the phosphate on the 3-position of the 3-phosphoglycerate is transferred to the hydroxyl residue at position 2. Removal of the elements of water from 2-phosphoglycerate results in the formation of an enolic phosphate compound, phospho(enol)pyruvate (PEP). The free energy of hydrolysis of PEP to form the enol form of pyruvate and Pi is on the order of −4 kcal/mol. In aqueous solution, however, the enol form of pyruvate is very unstable. Thus, the hydrolysis of PEP to form pyruvate is a very exergonic reaction. The ΔG0' for this reaction is −14.7 kcal/mol, which corresponds to an equilibrium constant of 6.4×1010. PEP is thus an excellent phosphoryl donor and the formation of pyruvate is coupled to ATP synthesis. Since two molecules of pyruvate are formed per glucose catabolized, two ATP are formed. Thus the net yield of ATP is two per glucose oxidized to pyruvate.
In some organisms, glycolysis is the only source of ATP. A familiar example is yeast growing under anaerobic (no oxygen) conditions. In this case, glucose is said to be fermented and ethyl alcohol and carbon dioxide (CO2) are the end products (Fig. 5). In contrast, all higher organisms can completely oxidize pyruvate to CO2 and water, using molecular oxygen as the terminal electron acceptor. The conversion of glucose to pyruvate releases only a small fraction of the energy available in the complete oxidation of glucose. In aerobic organisms, more than 90% of the ATP made during glucose catabolism results from the oxidation of pyruvate.
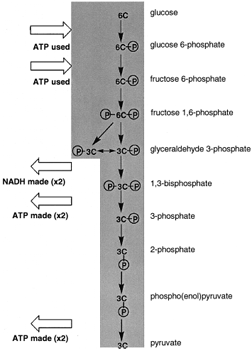 |
| Figure 4 A view of glycolysis. Glucose, a six-carbon sugar, is cleaved and oxidized to two molecules of pyruvate. There is the net synthesis of two ATP per glucose oxidized and two NADH are also formed. |
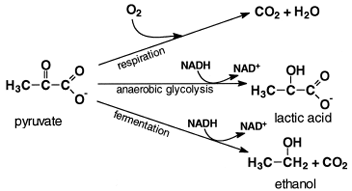 |
| Figure 5 Fates of pyruvate. In yeasts under anaerobic conditions, pyruvate is decarboxylated and reduced by the NADH formed by glycolysis to ethanol. In anaerobic muscle, the NADH generated by glycolysis reduces pyruvate to lactic acid. When O2 is present, pyruvate is completely oxidized to CO2 and water. |




