Determination of Sulphate and Sulphide in Water
B. DETERMINATION OF SULPHIDE
A. DETERMINATION OF SULPHATE
AimTo determine the amount of sulphate present in the given samples.
Principle
Sulphate is widely distributed in nature and may be present in natural water in concentrations ranging from a few to several thousand milligrams/litre. Sulphates are of considerable concern because they are indirectly responsible for two serious problems often associated with the handling and treatment of wastewater. Odour and sewer corrosion problem result from the reduction of sulphates to hydrogen sulphide under anaerobic conditions.
- Gravimetric method with ignition of residue.
- Gravimetric method with drying of residue.
- Turbidimetric method.
1. Gravimetric Method with Ignition of Residue
Principle
Sulphate is precipitated in hydrochloric acid medium as barium sulphates by the addition of barium chloride. The precipitation is carried out near the boiling temperature and after a period of digestion the precipitate is filtered; washed with water until free of chlorides, ignited and weighed as barium sulphates.
- Drying oven
- Desiccator
- Steam bath
- Analytical balance
- Ashless filter paper (Whatman filter paper No. 42)
- Muffle furnace
- Glassware like funnel, flask and pipette
Reagents (click to check the preparation of reagents)
- Methyl red indicator solution
- Hydrochloric acid
- Barium chloride solution.Gravimetric method with drying of residue.
- Silver nitrate-nitric acid reagent
- Take 250 mL of the sample in a conical flask.
- Adjust the acidity with HCl to 4.5 to 5 using a pH meter or the orange colour of methyl red indicator.
- Then add an additional 1 to 2mL HCl.
- Heat the solution to boiling and while stirring gently, add barium chloride solution slowly until precipitation appear to be completed. Then add about 2 mL in excess.
- Digest the precipitate at 80°C to 90°C preferably overnight but for not less than 2 hours.
- Filter the contents in the flask through an ashless filter paper.
- Wash the precipitate with small portion of warm distilled water until the washing is free of chloride as indicated by testing with silver nitrate-nitric acid reagent.
- Place the precipitate along with filter paper in a crucible after finding its empty weight and dry it.
- Keep the crucible in a muffle furnace and ignite at 800°C for 1 hour.
- Cool in a desiccator and weigh.
- Find weight of the barium sulphate precipitate.
2. Gravimetric Method with Drying of Residue
If organic matter is not present in the sample, first method can be done without igniting and instead drying the residue and weighing.
Principle
The turbidimetric method of measuring sulphate is based upon the fact that barium sulphate tends to precipitate in a colloidal form and that this tendency is enhanced in presence of a sodium chloride-hydrochloric acid solution containing glycerol and other organic compounds. The absorbance of the barium sulphate solution is measured by a nephelometer or turbidimeter and the sulphate iron concentration, determined by comparison of the reading with a standard curve.
Apparatus
- Nephelometer or Turbidimeter
- Magnetic stirrer
- Stopwatch
- Measuring spoon 0.2 to 0.3 mL capacity.
- Conditioning agent
- Barium chloride
- Standard sulphate solution
Procedure
- Measure 100 mL or suitable portion of the sample into a 250 mL Erlenmeyer flask.
- Add 5 mL of conditioning reagent and mix it by placing on a magnetic stirrer.
- Add a spoonful of barium chloride crystals and begin timing immediately.
- Stir at constant speed exactly for one minute.
- After stirring pour some of the solution into the absorption cell of the photometer, and measure the turbidity at 30 second intervals for four minutes.
- Usually maximum turbidity occurs within two minutes and the reading remains constant thereafter for 3 to 10 minutes. So, take reading with maximum turbidity occurring in within four minutes.
- Prepare a calibration curve. The standards are prepared at 5 mg/L increments in the 0-40 mg/L sulphate range and their turbidity or absorbance read.
- Absorbance versus sulphate concentration is plotted and a curve is obtained.
- Finding the absorbance for a given sample, the concentration of sulphates in the solution is determined with the help of calibration curve.
Observation
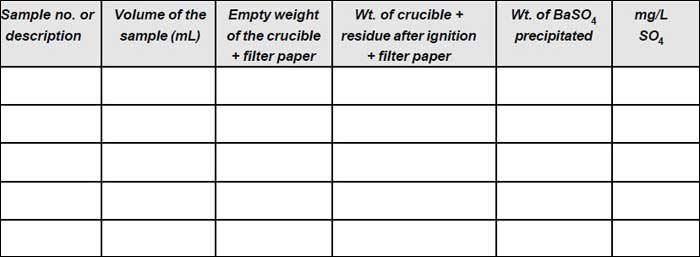
Weight of filter paper =...........
Calculation
| SO4 in mg/L = | mg of BaSO4 | x 411.6 = ............. |
| mL of sample |
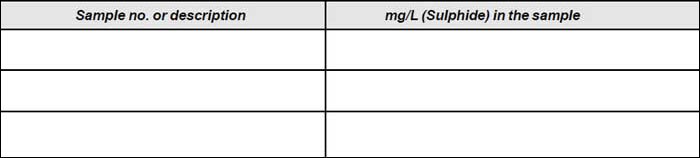
Result
B. DETERMINATION OF SULPHIDE
To determine the amount of sulphide present in the sample by titrimetric method.
Principle
Sulphides often occur in ground water especially in hot springs, in wastewater and polluted waters. Hydrogen sulphide escaping into the air from sulphide containing wastewater causes odour nuisance. It is highly toxic and cause corrosion of sewers and pipes. Sulphides include H2S and HS– and acid soluble metallic sulphides present in the suspended matter. Iodine reacts with sulphide in acid solution, oxidising it to sulphur; a titration based on this reaction is an accurate method for determining sulphides at concentration above 1mg/L if interferences are absent and if loss of H2S is avoided.
- Burette
- Pipette
- Erlenmeyer flask.
Reagents (click to check the preparation of reagents)
- Hydrochloric acid
- Standard iodine solution (0.025N)
- Standard sodium thiosulphate solution (0.025N)
- Starch solution
Procedure
- Measure from a burette 10mL of iodine into a 500 mL flask.
- Add distilled water and bring the volume to 20 mL.
- Add 2 mL of 6 NsHCl.
- Pipette 200 mL sample into the flask, discharging the sample under the surface of solution.
- If the iodine colour disappears, add more iodine so that the colour remains.
- Titrate with sodium thiosulphate solution, adding a few drops of starch solution, as the end point is approached and continuing until the blue colour disappears.
Observation
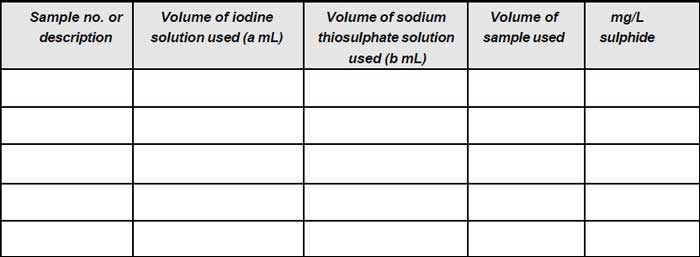
Calculation
| mg/L sulphide = | 400 (a – b) |
| mL of sample |
where,
a = mL 0.025 N iodine used
b = mL 0.025 N sodium thiosulphate solution used.
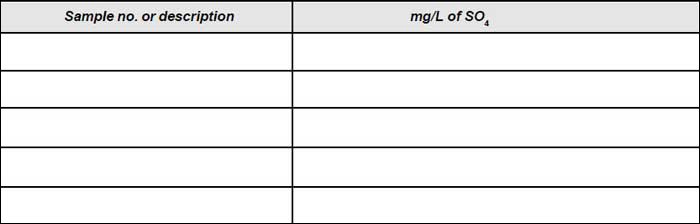
Result