Secondary Metabolites
In the beginning of 20th century, the idea of growth inhibition of one microorganism present in the vicinity of other one came into existence. Later, it was demonstrated that growth inhibition of the former micro-organism was mediated by secretion of toxic metabolites by the later. This toxic metabolite was termed as 'antibiotic' and the phenomenon of act of growth inhibition by antibiotics as 'antibiosis'. The antibiotics are defined as "the complex chemical substances, the secondary metabolites which are produced by microorganisms and act against other microorganisms".
However, antibiotics produced by microorganisms have been very useful for the cure of certain human diseases caused by bacteria, fungi and protozoa. Due to continuous endeavour made in this field, the antibiotics discovered at present are about 5,500. Total world production of antibiotics is more than one million tonne per annum. This success has been possible only due to continuous researches made during the last 4 decades. A list of microorganisms producing antibiotics and their applications are given in Table 16.2.
Table 16.2. Antibiotics produced by microorganisms.
| Microorganism | Antibiotics* | Applications |
| Bacteria : | ||
| Bacillus brevis | Tyrothricin (G+, G-) | Mouth and throat infection |
| B. polymyxa | Polymyxin B (AT) | UTI, gastroenteritis |
| B. subtilis | Bacitracin (G+) | Dermatitis, superficial pyogenic infection, dysentery |
| Streptococcus cremoris | Nisin (G+) | In cheese, food preservation (non-medical use) |
| Actinomycetes: | ||
| Micromonospora purpurea | Gentamicin (G+, G-) | UTI, abscess |
| Nocardia, mediterranei | Rifamycin (My) | Meningitis |
| Streptomyces griseus | Streptomycin (G+, G-, My) | Tuberculosis |
| S. aureofaciens | Tetracyclines *(G+, G-) | Cholera, tetanus, UTI |
| S. erythreus | Erythromycin (G+) | Cholera, tetanus, arthritis |
| S. noursei | Nystatin (AF) | Skin lesions |
| S. spheroides | Novobiocin (G+) | Abscess |
| Fungi: | ||
| Cephalosporium acremonium | Cephalosporins (G+, G-) | UTI, pneumonia, meningitis |
| Penicillium chrysogenum | Penicillin (G+) | Pneumonia, pharyngitis |
| P. griseofulvum | Griseoftilvin (AF) | Skin and hair lesions |
| P. notatum | Penicillin (G+) | Fever, pneumonia, genital infection |
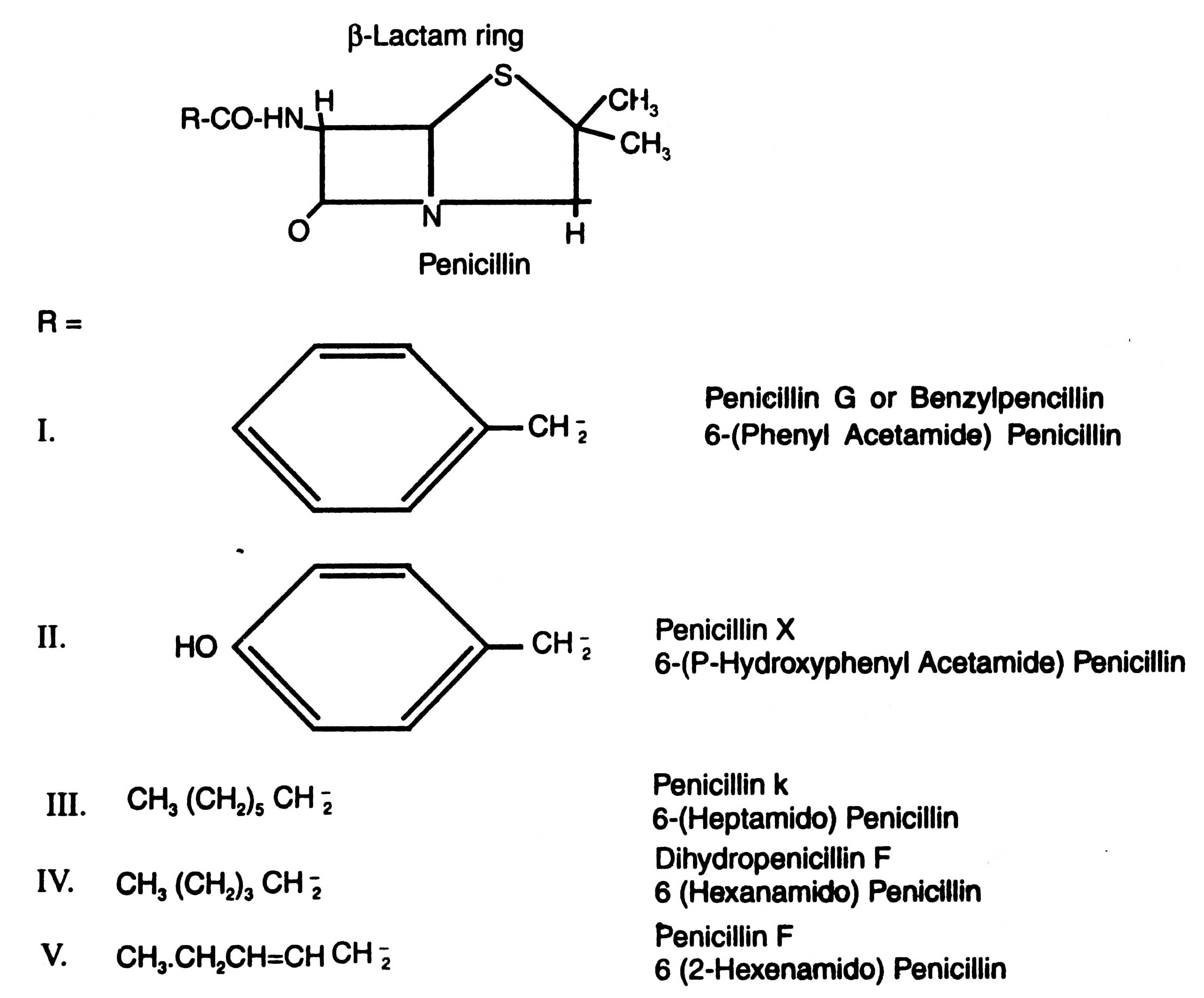
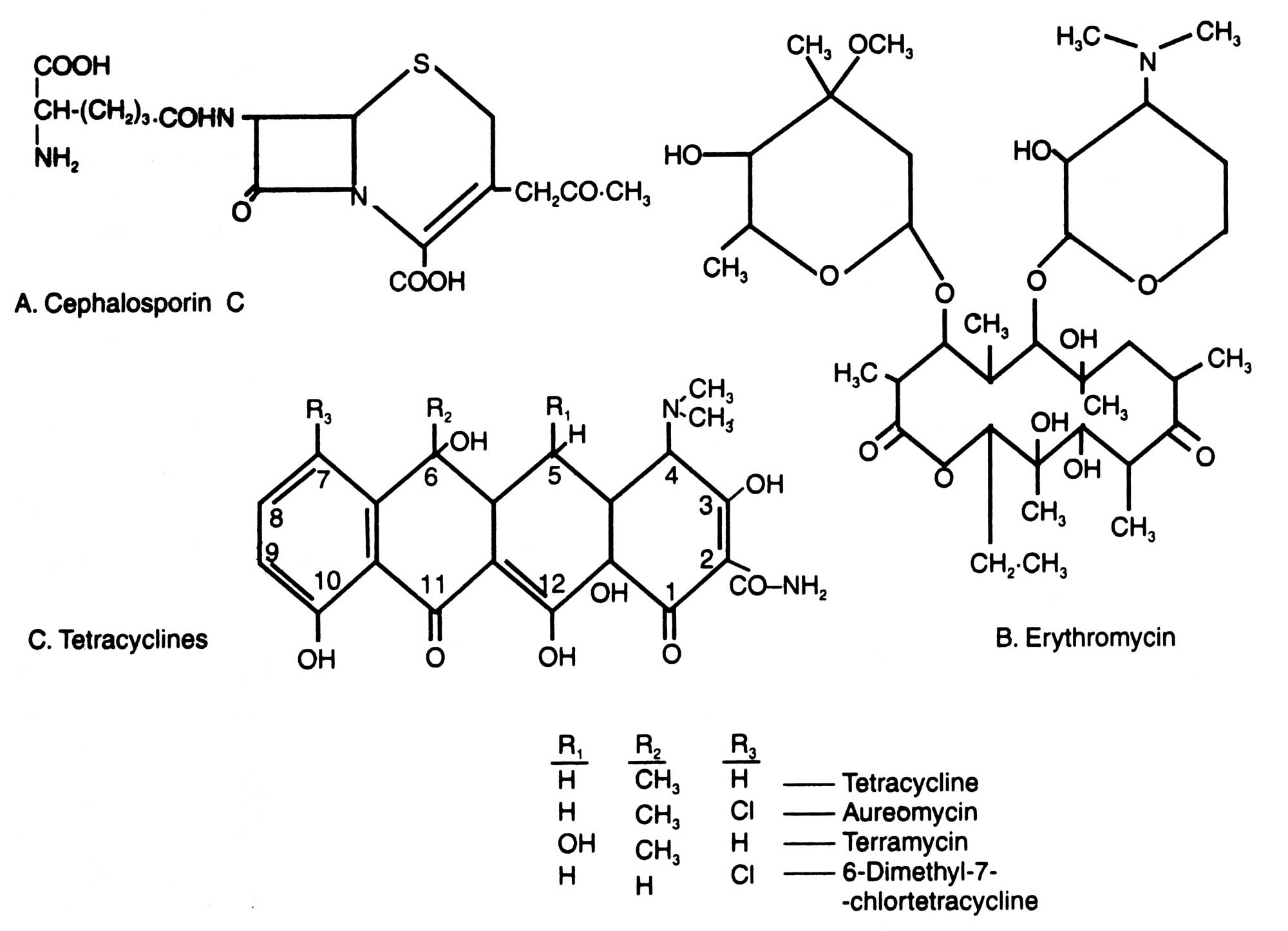
Among the antibiotics discovered so far, there are 4 major groups which are most extensively used throughout the world : the penicillins (Fig. 16.2), cephalosporins, tetracyclines and erythromycins (Fig. 16.3).
Moreover, researches done on this aspect have shown (in U.K.) that the synthesis of some of the antibiotics in Streptomyces was mediated not by a plasmid. Therefore, there is possibility to produce new antibiotics by transfer of plasmids into a single cell of Streptomyces.
Penicillins
It was Alexander Fleming (1929) who first discovered the bacteriostatic principle from a fungus and named it penicillin. He observed that a fungal contaminant prevented the growth of staphylococci, which was later on identified as Penicillium notatum. Clutterbuck et al, (1932) studied the chemical nature of penicillin. They found that penicillin was an organic acid which was dissolved into organic solvents from aqueous solutions at low pH. It was vulnerable to hydrogen ion (H+) and heat. After evaporation of solution to dryness, the biological activity was lost. Further studies done on P. notatum confirmed that this mould could produce about 2 ppm active substance.
The biological activity and recovery of penicillin were investigated by Chain et al, (1940). They cultured Fleming's fungus in surface culture in a small pilot plant scale and recovered penicillin in 1,000 fold amount only by keeping low temperature during the extraction. They also produced dry powder in the form of salt of penicillin.
This was the first attmept to extract penicillin salt in the form of dry powder which could have the curative properties.
It was the time of World War II when significance of penicillin was realized. England had no money to expend on penicillin production. Thus, the Oxford group was in crisis. Dr. Florey and Dr. Heatley came to U.S.A. America took up the problem and gave high priority on antibiotic production. This is why, by the time of attack of France in 1944, an adequate amount of penicillin was available to save the life of wounded people (Perlman, 1979).
Strain improvement
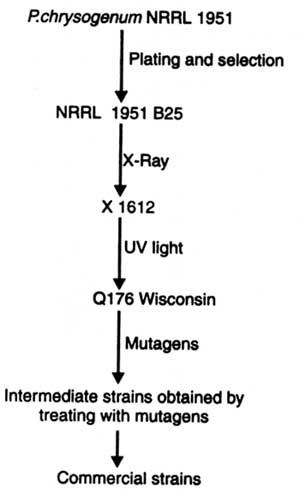
The fungus P. notatum originally used by Fleming for penicillin production, gave poor result. Moreover, many strains of this fungus were developed which produced many fold more penicillin than the original one. Besides P. notatum, P. chrysogenum was also tested which gave good results in submerged culture condition. One of these strains was P. chrysogenum NRRL 1951. Mutations in P. chrysogenum are generally induced by ultraviolet (UV) radiation or other mutagenic chemicals (e.g. N-methyl-N-nitro-N-nitrosoguanidine (NTG) The subsequent strains isolated from NRRL 1951 by treating with mutagens are shown in Fig. 16.4.
The subsequent selected strains produced penicillin in increased amount. The concentration of penicillin was described in ‘Oxford units' i.e. the amount which inhibited growth of the Oxford strain of Staphylococcus aureus. To estimate the activity of penicillin, it is necessary that penicillin should be in highly purified and crystalline form. Thus, one Oxford unit is equal to 0.6 g/ml of pure crystalline penicillins, for example, sodium salt of benzylpenicillin. The efficiency of strains of P. chrysogenum producing penicillin was in the order : P. chrysogenum NRRL 1951 (80-100 units/ml)> NRRL B25 (100 - 200 units/ml)> x 1612 (300 - 500 units/ ml)> Q 176 (Wisconsin) (800 - 1000 units/ml)> intermediate strains (1,500-2,500-5000 units/ml)> commercial strain (10,000 units/ml). Therefore, the technological improvement and selection of strains yielded penicillin to about 20 g/liter which was about 10,000 times more than what was obtained from the strain producing penicillin in 1941. All of today's industrial strains were derived from the Wisconsin Q-176 and Wisconsin 51-20 improved strains.
The chemical nature of penicillins
Molecular structure of penicillins reveals that they include the 4 membered b-lactam ring. The amide bond of b-lactam ring is readily broken in both acidic and alkaline medium and can be hydrolyzed by penicillinase (b-lactamase) synthesized by many bacteria. The naturally occurring penicillins differ from each other in R groups. When different R group attaches with penicillin it results in different types of penicillin (Fig. 16.2).
Fermentation medium
For the first time, surface culture of P. notatum was carried out in Czapek-Dox broth. Rate of penicillin production increased on supplementing it with the organic materials such as yeast extract, casein or beef extract. In addition, increased yield was also obtained with amending the cotton seed, peanut meal and soybean meal (Chain, 1966). Several natural penicillins (e.g. penicillin F, penicillin K and dihydropenicillin F) are produced when a suitable medium is fermented by P. chrysogenum in the absence of supplemented precursor.
Production of these natural penicillins is also stimulated by the presence of fatty acids in the medium. Medium supplemented with phenylacetic acid, phenylacetamide or b-phenylethylamine stimulate the production of penicillin G (Lowe, 1986). Calam and Hockenhull (1949) developed a chemically defined medium which supported production of antibiotic by many strains of P. chrysogenum. Chemical composition of media is given in Table 16.2. Production of antibiotic was also increased by (i) keeping the pH of fermentation medium between 6.8 and 7.4, (ii) adding buffering agents e.g. CaCO3 and phosphate to the medium and sterile H2SO4 and NaOH when required, (iii) keeping the temperature at 25 ± 0.5°C during incubation, and (iv) agitating the culture for aeration in a large fermenter.
Fermentation process
Penicillins are produced on large scale in a commercially devised fermenter which provides optimum growth conditions to P. chrysogenum for maximum yield. Following are the steps for fermentation of benzyl-penicillin i.e\ Penicillin G.
(ii) After 4 days, transfer the content of flask to another flask (4 liter capacity) containing 2 liters medium and incubate for 2 days as earlier.
(iii) Transfer the content to a stainless steel tank (800 liter capacity) containing 500 liter medium. This tank is equipped in such a way that it could provide the optimum conditions for fungal growth.
(iv) After 3 days, use the contents for inoculation of about 1, 80,000 liter medium kept in a fermenter (2,50,000 liter capacity). The later is equipped with automatic devices to optimum growth conditions (e.g. temperature, pH, O2, etc.)
(v) Filter the content of fermenter after 6 days incubation. Filtrate contains penicillin. Extract the penicillin into amyl-or butyl-acetate. From it transfer the penicillin into aqueous solvent by extracting with phosphate buffer.
Table 16.3. Chemical composition of fermentation medium for the production of penicillins.
| Constituents (g/l)** | C. H. medium (g/l)* | Calam's medium (g/l)** |
| Lactose | 30 | 35 |
| Glucose | 10 | 10 |
| Starch | 15 | - |
| Corn steep solids | - | 35 |
| Ammonium sulphate | 5 | - |
| Ethyl amine | 3 | - |
| Vegetable oil | - | 2.5 |
| Citric acid | 10 | - |
| Acetic acid | 2.5 | - |
| Phenyl acetate | 0.5 | - |
| KH2PO4 | - | 4 |
| CaCO3 | - | 10 |
From a butanol-water mixture crystallize the potassium penicillin G. Again purify potassium penicillin G before its use.
Antibiotic producing companies
| Astra-IDL Limited, 32/1-2 Cressent lower, Cressent Road, Bangalore - 560001. India |
| Byer (India) Ltd., Express Towers, Nariman Point, Bombay-400021. India |
| Biochem Pharmaceutical Industries, A - Aidum Building, I-Dhobi Talao, Bombay-400002. India |
| Cadila Chemicals Pvt. Ltd., Maninagar, Ahmedabad-380008 India |
| Glaxo India Ltd., Annie Besant Road, Bombay-400025. India |
| Hindustan Antibiotic Ltd., Pimpri, Pune-411018. India |
| Hoechst India Ltd., Hoechst House, Nariman Point, 193 Backbay Reclamation, Bombay-400021. India |
| John Wyeth (India) Ltd., Steelcrete House, Dinshaw Wacha Road, Bombay 400020. India |
| Maharastra Antibiotics & Pharmaceuticals Ltd., Nagpur. India |
| The U.P. Drugs & Pharmaceuticals Co. Ltd., Lucknow. India |
| Indian Drugs & Pharmaceuticals Ltd., Rishikesh. India |
| Pfizer Ltd., Express Towers, Nariman Point, Bombay-400021. India |
| Sandoz (India) Ltd., Sandoz House, Annie Besant Road, Worli Bombay-400021. India |
| Sarabhai Chemicals, Wadi, Vadodara, India |
| American Cyanamid, U.S.A. |
| China National Chemical Import & Export Corporation, China. |
| Glaxo Laboratories Ltd., England. |
| Hoechst AG, W. Germany. |
| Imperial Chemical Industries Ltd., England. |
| Pfizer, Inc., U.S.A. |
| Wyeth Laboratories, U.S.A. |