Primary Metabolites
Alcohols
For the first time, Louis Pasteur demonstrated the fermentation of sugar by microorganisms and their regulation as well. He found that the yeast cells produce 20 times more cell materials from sugar under aerobic conditions than under anaerobic conditions. He explained that oxygen inhibits fermentation process. Yeasts are aerobic microorganism, but glucose fermentation takes place under anaerobic condition. In the presence of oxygen yeasts increase its biomass (cell materials) but under anaerobic condition they hardly grow, however, ferment glucose very efficiently. Thus, oxygen supresses fermentation process. This is known as "Pasteur effect" as the inhibition of fermentation by air was described by Pasteur about 100 years ago. This effect is applicable for all facultative anaerobic microorganisms.After the U.S.A and Brazil, India is the worlds' third largest producer of fermentation ethanol. Now we have over 100 distilleries with an installed capacity of about 700 million liters per annum. Production of ethanol per tonne of molasses is about 225 liters.
There is a limited number of microorganisms which ferment carbohydrates (pentose or hexose sugars) into alcohols and yield some by-products. Microorganisms utilize various pathways. A summary of alcohol production through different routes of microorganisms is given in Fig. 15.4. Following are some of alcohol producing micro-organisms:
Bacteria: Clostridium acetobutylicum, Klebsiella pneumoniae, Leuconostoc mesenteroides, Sarcina ventriculi, Zymomonas mobilis, etc.
Fungi: Aspergillus oryzae, Endomyces lactis, Kloeckera sp., Kluyreromyees fragilis, Mucor sp., Neurospora crassa, Rhizopus sp., Saccharomyces beticus, S. cerevisiae, S. elltpsoideus, S. oviformis, S. saki,Torula sp., Trichosporium cutaneum, etc.

Fig. 15.4. Production of alcohols by microorganisms 1-Lactobacillus brevis; 2-Leuconostoc mesenteroides.
Fermentable Substrates
Ethyl alcohol is produced from such organic material that contains sugar or its precursor as fundamental units. The cost of substrates (raw materials) in fermentation is of major consideration, because it directly affects the cost of products. The fermentable substrates used for alcohol production are given under 'fermentable substrates' (see Fermentable substrate).
Before using in fermentation processes, the cellulosic, lignocellulosic and starchy materials are hydrolyzed by enzymes or acids just to render the complex substances into a simple forms (monosaccharides). Enzymes for hydrolysis are obtained from barley malt or moulds by heat treatment of acidified materials.
Clostridium acetobutylicum anaerobically ferments the starchy substrates of grains and potatoes to produce acetone, ethanol and butanol. Recently, it has been demonstrated that Schwaniomyces castellii directly converts the soluble starch into ethanol.
Biochemistry of Alcoholic Fermentation
In 1815, Gay-Lussac formulated the conversion of glucose to ethanol and carbondioxide (CO2). The Formula is given below:
C6H12O6
 2C2H5OH + 2CO2
2C2H5OH + 2CO2Yeast (S. cerevisiae) converts di-and oligo-saccharides through EMP pathway into pyruvic acid. In other micro-organisms routes deviate from glucose-6-phosphate to either pentose phosphate pathway or the Entner-Doudoroff pathway. Even the fate of pyruvic acid differs in different alcohol producing microorganism. Therefore, differences lie in the alcoholic products. In the present context, ethanol formation is discussed with the examples of yeasts and bacteria.
Yeasts, especially strain of S. cerevisiae are the main producer of ethanol. They have been used as a major biological tool for the formation of ethanol since the discovery of fermentation process by the time of L. Pasteur. During 1890s fermentation of froth was discovered in sugar solution on addition of yeast extracts obtained by its grinding. This was the first evidence for a biochemcial process of in vitro formation of ethanol in the absence of yeast cells. The extract supplied inorganic phosphate (Pi) which is incorporated in fructose-l:6-bisphosphate. Fructose- l:6-bisphosphate is accumulated due to lack of ATP utilization for energy requiring reactions in the cell free systems. Therefore, an excess of ATP is maintained. The reaction is given below:
2C6H12O6+Pi
 2C2H5OH +2CO2+2H2O+fructose-1:6-bisphosphate
2C2H5OH +2CO2+2H2O+fructose-1:6-bisphosphateThis equation is known as Harden - Young equation after the name of the discoverer. Energetics of EMP pathway reveals that one molecule of glucose yields only 2 molecules of
ATP from 2 molecules of ADP under anaerobic condition in contrast of 38 molecules of ATP
through respiration:
C6Hl2O6 + 2Pi + 2ADP
 2C2H5OH+2Co2+2H2O+2ATP + energy.
2C2H5OH+2Co2+2H2O+2ATP + energy.A total of 15.4 Kcal energy is evolved from one molecule of glucose as about 77 Kcal free energy is obtained from one molecule of ATP. Fermentation of carbohydrate is exothermic where certain amount of energy is lost to the environment as heat. However, temperature of fermenter gets increased. Increase in temperature (generally from 11-22°C) depends on size of the fermenter.
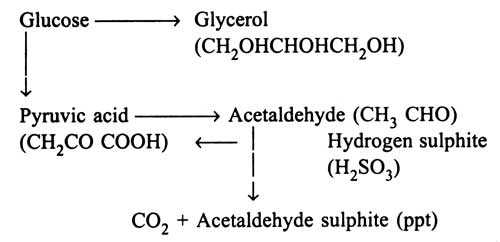
The chart shows that during fermentation of glucose, acetaldehyde, an intermediate product, is formed which can bewtrapped with hydrogen sulphite which is non-toxic to yeast cells. On addition of hydrogen sulphite, during fermentation, acetaldehyde sulphite is precipitated. This results in production of glycerol, by diminishing the yield of ethanol and carbon dioxide. Therefore, fermentation of glycerol has been developed industrially by incorporation of hydrogen sulphite.
Ethanol Formation by Bacteria
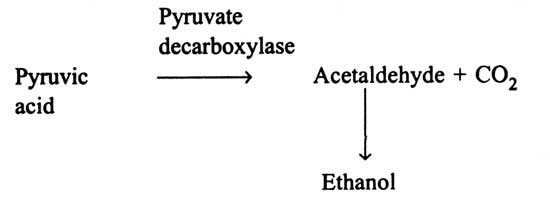
Among the bacteria discovered, only Sarcina ventriculi forms ethanol through fructose-1:6-bisphosphate pathway i.e EMP pathway and pyruvate decarboxylase as formed by yeasts. A rod shaped polarly flagellated and motile bacterium (Zymomonas mobilis) is known to metabolize glucose through the Entner-Doudoroff pathway (Fig. 14.4) and results in pyruvic acid. Pyruvic acid is then decarboxylysed by pyruvate decarboxylase to acetaldehyde and carbon dioxide. Acetaldehyde is reduced to ethanol. Thus, the fermentation products are ethanol, carbondioxide (and small amount of lactic acid). In some members of Enterobacteriaceae and Clostridia, ethanol is formed as a subsidiary product. Acetaldehyde is not directly produced from pyruvic acid by pyruvate decarboxylase, but originates through reduction of acetyl CoA.
Moreover, the hetero-fermentative lactobacilli (e.g. Leuconostoc mesenteroides) use quite different pathway for alcohol production (Fig. 15.4). In the beginning of fermentation they utilize pentose cycle to result in xylulose-5-phosphate, which is then cleaved by phosphoketolase into acetyl phosphate and glyceraldehyde-3-phosphate. Acetaldehyde dehydrogenase and alcohol dehydrogenase reduce the acetylphosphate into ethanol. Similarly, glyceraldehyde-3-phosphate is converted via pyruvic acid to lactic acid (Schlegel, 1986).
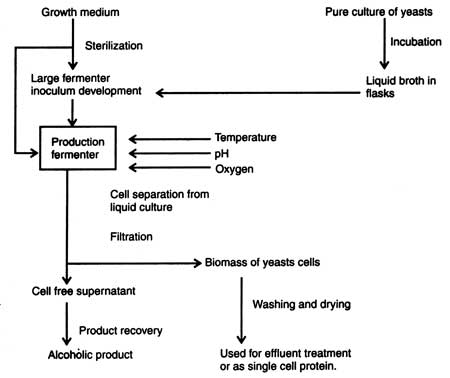
Fermentation of ethanol is carried out in a large fermenter (size 1000 to 1.5 million dm2). The inoculum of microorganism is maintained in fermenter at the optimum growth conditions such as temperature, pH, oxygen and concentrations of carbohydrate, the substrates. Before starting the fermentation pure inoculum (starter inoculum) of species of Saccharomyces is prepared by inoculating the well defined and sterilized medium (Fig. 15.5). At the same time fermentation medium is formulated, sterilized and transferred to the sterile fermenter. Liquid medium in fermenter is inoculated with a small amount of inoculum of yeast. Growth conditions of liquid broth is maintained to provide optimum conditions such as temperature, pH, oxygen, etc. for the production of ethanol.
After inoculation at optimum growth conditions for different periods the culture fluid is filtered when growth of the microorganism is over. Consequently, the yeast cells (biomass) are separated from the supernatant. From the supernatant products are recovered and purified. The yeast mass is used for effluent treatment or as the source of single cell protein.
Commercial production of alcohol varied in scale. Production and product recovery depend on (a) size of fermenter, (b) optimum culture conditions, (c) design for separation of solid and liquid, (d) culture medium, (e) potential microorganism, and (f) joint efforts of microbiologists, geneticists, biochemists, engineers and chemists. Productive microorganisms are improved through the improvement in growth medium, growth condition, mutation, recombination and process design.
Distilleries Producing Alcohol
There are over 127 distilleries in India, producing about 732mL of alcohol per annum. Some of the distilleries functioning in U.P. are given below:
| 1. | Awadh Sugar Mills Distillery |
| 2. | Baheri Asawani Distillery |
| 3. | Cooperative Sugar Factory Ltd. |
| 4. | Ajudhia Sugar Mills Distillery |
| 5. | Simbhaoli Sugar Mills Distillery |
| 6. | Saraya Distillery |
| 7. | Captanganj Distillery |
| 8. | Carew Corporation Ltd. Roza |
| 9. | Hindustan Sugar Mills Ltd. (Distillery) Gola |
| 10. | Dyer Meakin Breweries Ltd. |
| 11. | Standard Refineries and Distilleries Ltd. |
| 12. | Modi Industries Ltd. (Distillery) |
| 13. | Narang Distillery |
| 14. | P.V.K. Distillery |
Alcoholic Beverages
Percentage of alcohol differs, in different alcoholic beverages (Table 15.1). Some of them are briefly discussed:It is mainly an European drink produced from juice of fresh grapes (Vitis vinifera, V. rotundifolia). In ripen grapes, concentration of sugar (glucose and fructose) increases. Grape juice (27% sugar) is fermented by various strains of S. ellipsoideus into alcohol and also renders the chemical constituents which alters the flavor.
The fortified wines (brandy) are prepared on addition of extra ethanol to wines, when fermentation is over, for raising the concentration to about 20%.
Beer
Beer is produced after the fermentation of mixture of barley malt and starchy solutions by S. cerevisiae (a top fermenter yeast that do not settle at bottom) or S. carlsbergensis (the bottom fermentation yeast).
After fermentation sugar is converted into alcohol and also brings about minor chemical changes, for example protein.
Table 15.1. Alcoholic contents in some beverages.
| Beverage | Substrates | Alcohol(%) * |
| Beer | Cereals | 4-8 |
| Wine | Grape juice | 10-22 |
| Cidar | Apple juice | 8-12 |
| Champagne | Grape juice | 12-13 |
| Brandy | Wine | 43-57 |
| Whiskey | Cereals | 51-59 |
| Rum | Molasses | 51-59 |
| Gin | Cereals | 51-69 |
Rum is the distilled product of culture fluid. S. cerevisiae or other yeast is used as the fermenting microorganism. Culture medium is prepared from black strap molasses containing 12-14% fermentable sugar. Ammonium sulphate and some times phosphates are added as nutrients. When fermentation is over, culture fluid is distilled to remove the alcohol and used as rum.
Whiskey
It is prepared through the fermentation of grain mash (cooked and saccharified with peated malt) by a top yeast (S. cerevisiae) when fermentation is over, the culture fluid contains alcohols, traces of acids and esters.
Sake
Sake, the rice wine, is manufactured from the strach. It is a complicated process which implies the mastering of different fermentation techniques in semi-solid and sub merged conditions, and regulation of successive microbial populations. First of all a mould (Aspergillus oryzae) then bacteria (Lactobacillus and Leuconostoc) and finally yeast (5. cerevisiae) are mixed with the fermentable medium. The culture fluid contains about 20% alcohol; therefore, before marketing the concentration of alcohol is adjusted to 16% (Sasson, 1984).
(a) Ethanol is used as solvent, extractant and antifreeze. It is also used as a substrate for the synthesis of many other solvents of dyes, Pharmaceuticals, lubricants, detergents, pesticides, plasticizers, explosives and resins, and for the manufacture of synthetic fibers (Sasson, 1984). It has also been used as liquid fuel with the name 'gasohol'.
(b) N-butanol is used in the manufacture of plasticizers, brake fluids, urea-formaldehyde resins, extractants and pertrol additives.
(c) Glycerol is used in medicals, biosynthesis of D-fructose via mannitol, and in food industry
(because of its sweetness and high solubility). Mannitol is used in industry and research.
(d) Butanol plus acetone acid 2, 3-butanediol are used as industrial solvent. Butanol plus acetone is used in the production of explosive materials (e.g. cordite) and 2, 3-butanediol in the synthesis of rubber.
(e) Ethanol is used as alcoholic beverages as described earlier.