Vaccines for Hepatitis B Virus
Although the whole viral genome has been cloned and sequenced, yet there is limited information about amino acid sequence of surface and core antigens. Recently, HBV DNA has been successfully cloned in E. coli and mammalian cells, and synthesis of HBsAg and HBcAg particles has been done in the cells. Burrell et al. (1979) inserted HBcAg genes inPBR322 near b-glactosidase gene (Edman et al., 1981). Production of these genes is needed in order to get production of vaccines on a large scale. In yeast or mammalian systems, these antigens are synthesized more efficiently than in prokaryotes.
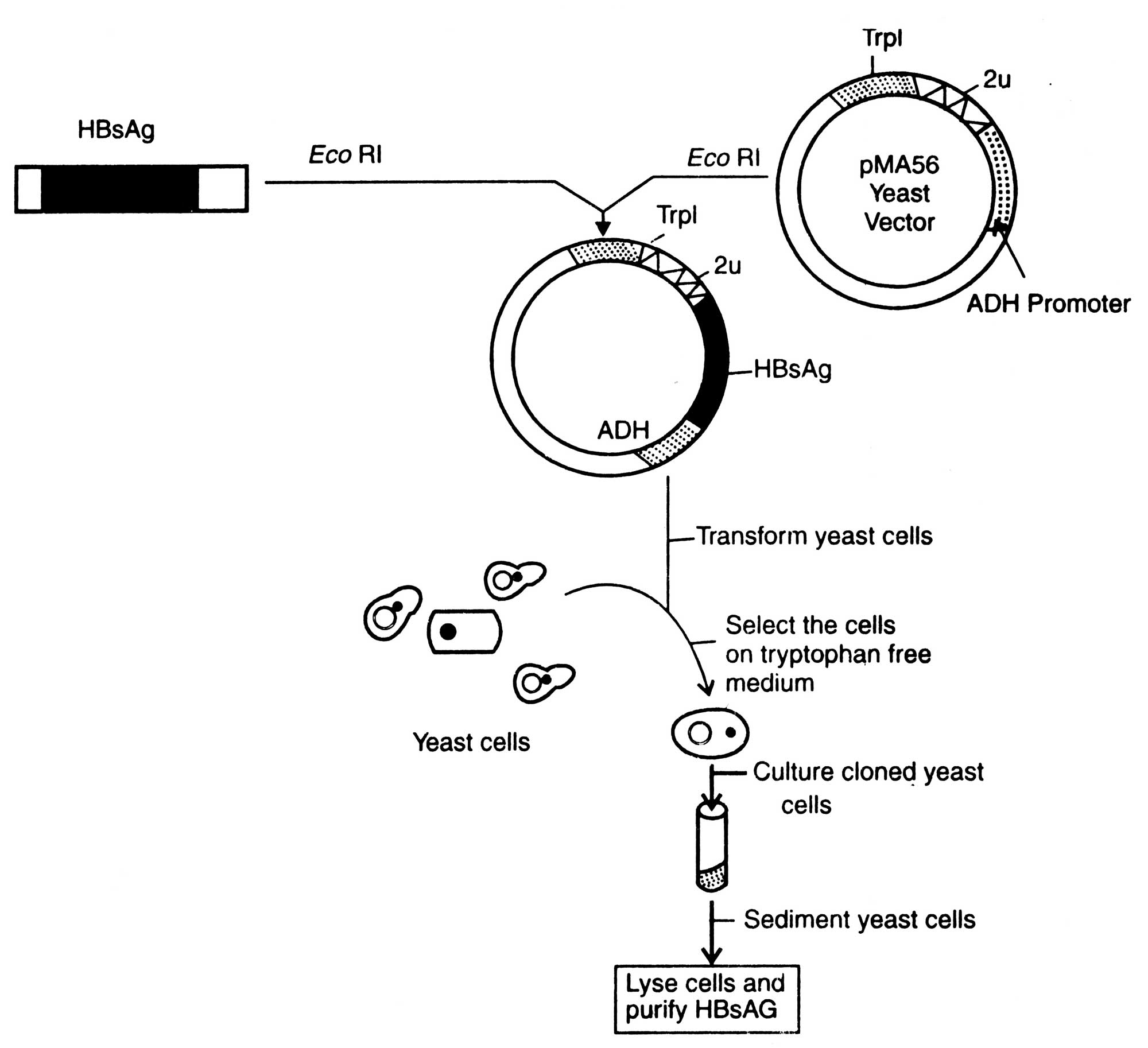
(i) Recombinant vaccine for Hepatitis B virus. After infection, HBV fails to grow and even in cultured cells it does not grow. This property has been explained to be due to inhibition of its molecular expression and development of vaccines. Recombinant vaccine for HBV was produced by cloning HBsAg gene of the virus in yeast cells. The yeast system has its complex membrence and ability of secreting glycosylate protein. This have made it possible to build an autonomously replicating plasmid containing HBsAg gene near the yeast alcohol dehydrogenase (ADH) Ipromoter (Fig.5.5). The HBsAg gene contains 6 bp long sequence preceding the AUG that synthesizes N-terminal methionine. This is joined to ADH promoter cloned in the yeast vector PMA-56.
(ii) Indigenous Hepatitis-B vaccine. India's first genetically engineered vaccine (Guni) against HBV developed by a Hyderabad based laboratory (Shantha Biotechnics Pvt. Ltd.) was launched on August 18, 1997. India is the fourth country (after the U.S.A., France and Beligum) to develop this highly advanced vaccine. The indigenous yeast-desired HBV vaccine is one third the cost of the imported vaccine. This new vaccine had undergone human clinical trials at Nizam's Institute of Medical Sciences, Hyderabad and K.E.M. Hospital, Mumbai. The clinical trials clearly proved that the seroprotection is about 98%. It was found more effective than the imported vaccine. The Drug Controller General of India has permitted it for commercial manufacture.