Methods of gene transfer
(i) Virus vectors.
After 1980, much work has been done on retroviruses as gene transfer vectors, more specifically on murine-leukemia virus (MLV) for gene therapy. Efforts are being made to develop HIV-based vectors so that even non-dividing cells can be injected. The outline of retroviral gene transfer has been shown in Fig. 5.8. The steps of developing a replication-defective recombinant retroviral vectors are : (i) the replacement of viral structural genes e.g. gag, pol and env by the therapeutic foreign genes of interest, (ii) transfection of this vector into packaging cell line (i.e. producer cells) that provide the viral structural proteins in trans so that the recombinant retroviral genome is packed and replication defective retroviruses are generated, (iii) transfection of host cells by such viruses, and reverse transcription of recombinant retroviral RNA and random integration into the host genome. In the absence of viral genes, the foreign genes (therapeutic in nature is transcribed from the viral LTRs, the long terminal repeats) and desired protein is synthesized (Fig. 5.8). The retroviral vectors are used in ex vivo gene transfer experiment, although it has been shown that they can infect a regenerating liver when administered intravenously into hepatectomized animal.
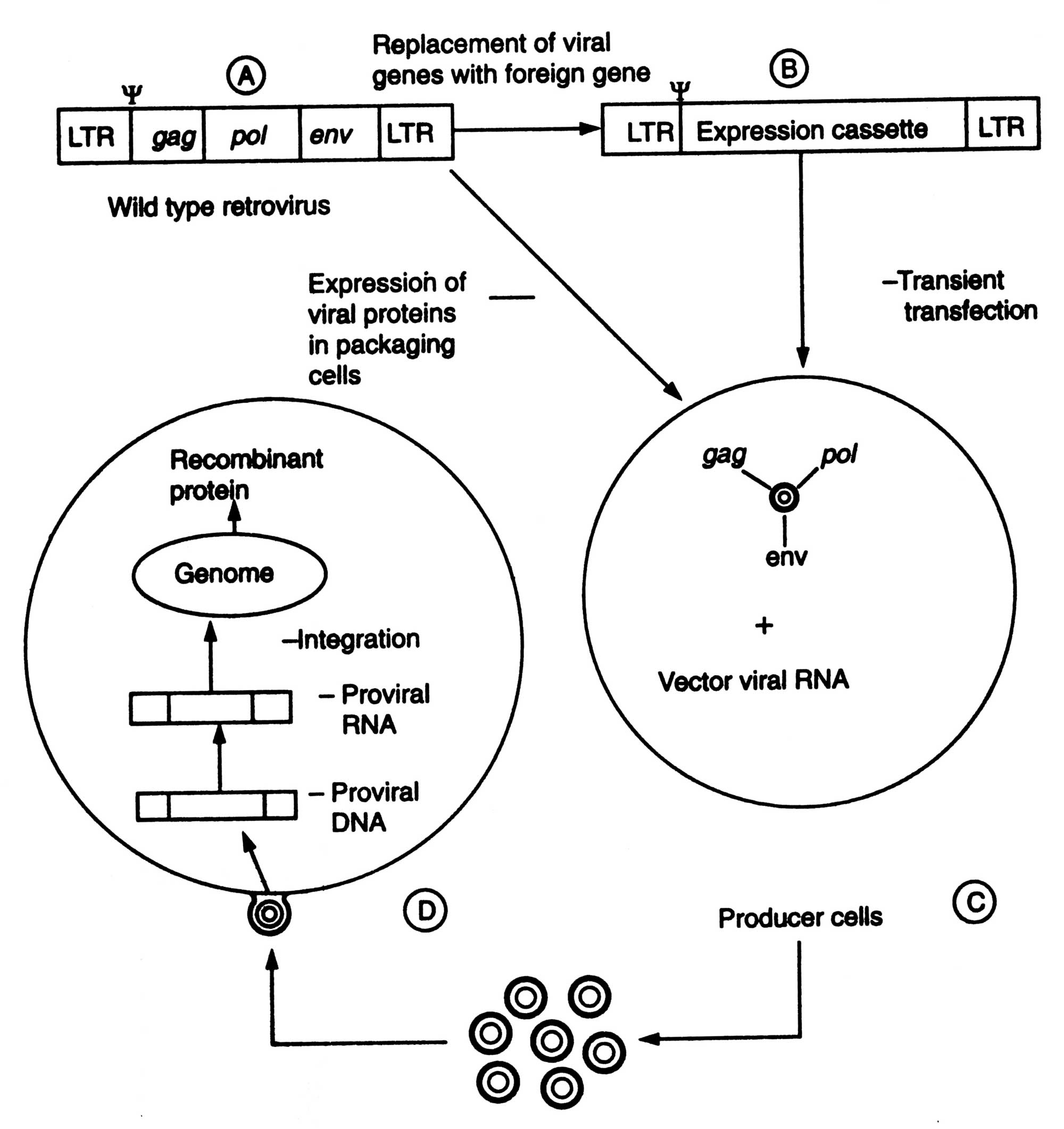
Fig. 5.8. Retroviral gene transfer A. Construction of a retroviral vector; B. Production of recombinant retrovirus; C. Transfection of target cells; D. Synthesis of recombinant protein.
Live adinoviruses have been used as vaccine in US military personnel without any major side effects. These can infect a wide range of cell types. Adinoviruses are the attractive candidates for the therapy of lung disorders because of their natural tropism for infecting respiratory epithelium. Unlike retroviruses, they can infect post mitotic cells as well.
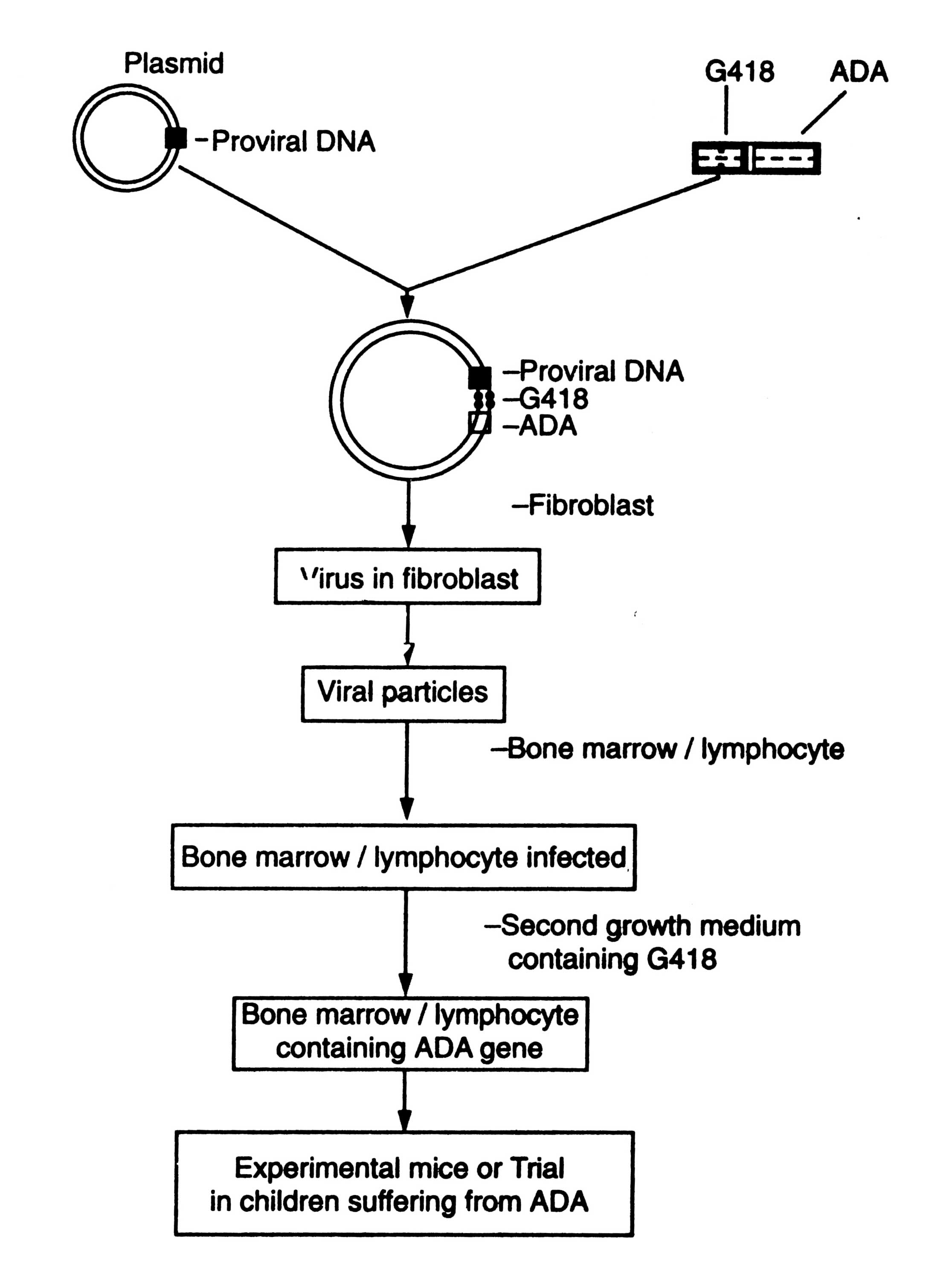
Adino-associated virus (AAV) is a non-pathogenic parvovirus of humans. It has aroused interest as a vector for gene therapy. It requires a helper virus co-infection for viral replication. The potential use of this virus as a gene transfer vector has intensified research on the life cycle of the virus. An outline of introduction of ADA-gene into the bone marrow cells/lymphocytes is given in Fig. 5.9. ADA-gene is fused with an antibiotic G418.
(ii) Non-viral approaches.
Most of approaches in gene therapy have been focused on development of viral vectors rather than clinical efficacy and biosafety. Furthermore, techniques of virus-based gene therapy is very costly and complex. Therefore, this led to the development of non-viral gene therapy. Majority of non-viral methods follow the in vivo approaches. Therefore a gene can be used successfully as a drug. In general, the non-viral methods for gene transfer can be grouped into two methods, physical and chemical methods.
The various forms of physical methods are given below :
Microinjection : It is a very tedious method. However, it is used in oocytes, eggs, and embryos. Jeffrey S. Chamberlain et al. (1993) of Human Genome Center, Michigan University U.S.A. have cured mice that inherited a neuro-muscular disease which is similar to muscular dystrophy of humans (for detail see 3.2.2.4). The mutant mice lacking 427 K gene in brain and muscle cells had a weak diaphragm which gradually degenerates like that in dystrophy affected humans. Chamberlain and coworkers microinjected the DNA containing 427 K gene into the zygote of mutant mice. The transplanted genes worked properly and produced necessary protein and thus, prevented the diaphragm.
Direct DNA injection.
Direct injection of DNA into skeletal muscle has created a lot of excitement and led to the possibility of using gene as vaccines. Direct gene transfer was attempted since early 1960s but these studies were not done seriously due to inefficient gene transfer and low level of expression. Due to low level of expression therapeutic benefits for the treatment of genetic disorder could not be derived.
Thereafter, these studies were further carried out. The low levels of foreign proteins synthesized in the host cells were processed along the class I MHC pathway leading to presentation of peptides on cell surface resulting in a potent immune response against the foreign antigens. This gave the birth to the concept of DNA vaccine or genetic immunization (Rangarajan and Padmanaban, 1996) (see DNA Vaccines).
It was developed originally for transformation of plant cells, but in animal system it is also being used for delivery of genes into tissues such as liver, skin, mammary glands and muscle. Direct gene delivery is now being used to map promoter elements that regulate gene expression in vivo, in transfection of herpes simplex thymidine kinase genes into melanomas to render them sensitive to gancyclovir and as a vaccination strategy. Thus, direct gene transfer technique failed to make an impression in treatment of genetic disorders but made enormous impact in the field of vaccine development. The other physical methods e.g. microinjection, electroporation, etc. have the limited scope in humans.
Magic bullets - cells as drug delivery system.
Magic bullet is a drug delivery system through a cell loaded with an appropriate treatment molecule which upon injection into a patients migrates through the circulatory system directly to the diseased site. Still, it is only a dream to the patients suffering from genetic diseases. In future it may be possible to cure such diseases such as cystic fibrosis, when a cell can reach to the lung tissues and deliver an enzymes to breakdown the thick getatinous mucus, and help in breath and food digestion.
Some times chemicals are used to make easy delivery of DNA into recipient cell.
Using detergent mixture. Certain charged chemical compounds e.g. calcium phosphate, dextran or lipids are mixed with functional cDNA of desired function. The mixture is introduced near the vicinity of recipient cells. Thereafter, it is spread to the interior of the body organ. The chemical mixed with cDNA disturbs the cell membranes, widens pore size and allows the cDNA to pass into the cell. In this method the number of cells allowing the entry of cDNA is very small.
Receptor-meditated gene delivery.
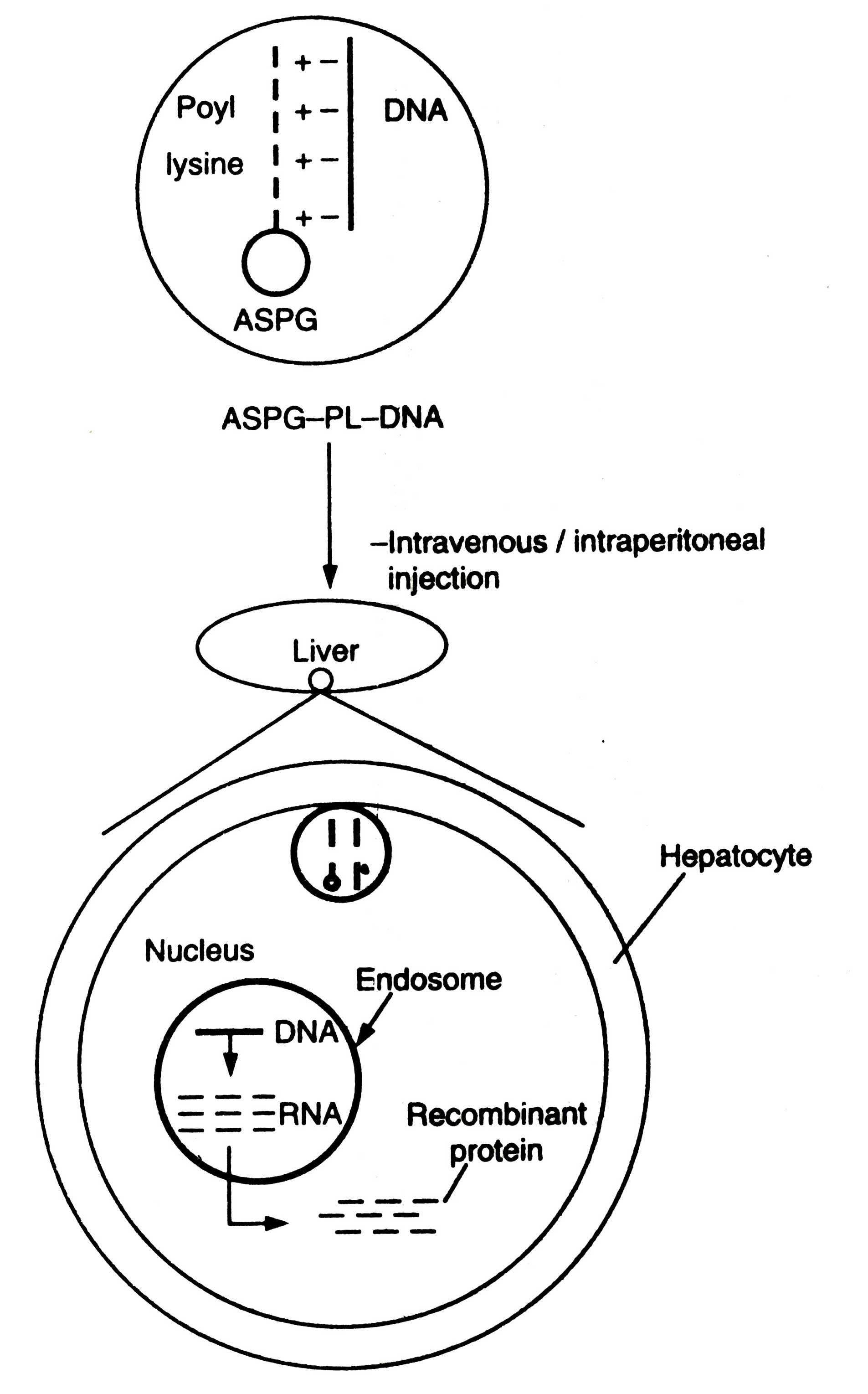
It is an attractive strategy. It takes advantage of normal physiological pathway. The receptors are exclusively present on hepatocytes. They bind to certain glvcoproteins lacking the terminal asialic acid. The concept of delivering genes into specific tissue was examined extensively with the liver asialoglycoprotein receptors. An asialoglycoprotein (orosomucoid) is coupled with poly-L-lysine. This conjugate is then condensed with plasmid DNA via electrostatic interactions (Fig. 5.10). This soluble complex i.e. asialoglycoprotein-poly-
Wu et. al. (1991) later extended the other cell surface receptors such as transferrin receptor. The major problem related to receptor mediated gene delivery system has been the degradation of DNA in the endosomes by lysosomal enzymes. Therefore, co-administration of lysosomotropic agents e.g. chloroquine is done to enhance gene expression. Moreover, it is established fact that adinoviruses escape lysosomal degradation by acidification of endosomes.
It has also been found that positively charged liposomes of cationic lipids can entrap the negatively charged DNA. This has led to the possibility of their use as gene transfer vehicle in vivo. Therefore, several catonic lipid formation have been developed and now being used for transfection of cells in culture and a variety of organs in vivo (Zhn et al, 1993). The two potential success of catonic lipids are: the aerosol delivery of genes to lungs, and in the development of cancer vaccines.
Embryo therapy through IVF-technology.
In 1993, adopting IVF-technology, Dr. A. Handyside (a team leader of British Researcher at Hammersmith Hospital, London) got success in producing a genetically engineered female baby, whose parents transmitted the genetic disease (cystic fibrosis) in earlier four babies, who died later on. Even after confirmation through prenatal diagnosis that the babies would be sufferer or carrier of this disease they decided to give birth to babies, which failed. This disease clogs the lungs of sufferer and makes them unable to digest the food properly.
Dr. Handyside admitted seven couples. Their eggs were collected and fertilized with their respective husbands'.sperm in test tubes. After 3 days incubation in vitro, when embryo reached 8 cell stages, a single cell was removed for analysis. The cell was magnified one million times by using a magnifying Thermal Cycler. The individual defective gene which causes cystic fibrosis was identified and screened out. Thereafter, the embryo was implanted in the womb of mother. Consequently, a female baby (C. O'Brien) was born who is neither sufferer nor carrier of these diseases, but a normal one. The birth of this genetically engineered baby has increased the hope of millions of couples who transmit the genetic disease to their offsprings, and also of researchers to look upon many more genetic diseases to cure.