Embryo Transfer
In 1890, the first case of developing pregnancy in rabbits through embryo transfer is known in literature. During 1930s the same method was used in goat and sheep. In catties cases of embryo transfer were reported after 1950 (BIOTOL series, 1992). Embryo transfer method cannot be used widely because of its high cost, technical difficulties and limited supply of embryo from superovulated donors. The embryo develops in foster mother (recipient) which simply acts as incubator and does not make any genetic contribution to the offspring.
Secondly, ruminant female carries one pregnancy at a time as only one egg is produced and fertilized with male's sperm. Thus, there is a chance for increasing the number of egg production at a time and transfer of fertilized eggs i.e. embryo into uterus of less important foster mothers other than original female in farm animal.
Multiple ovulation (Superovulation)
The reproductive cycle of ruminant female is such that the ovarian follicle of a non-pregnant female matures and releases single egg at a time. The time of ovulation differs in different animals, for example 21 days in cows and horse, 16 days in sheep and goats, etc. Normally ovulation occurs as a result of circulation of gonadotropic hormone. But by increasing the concentration of hormone the number of egg production gets increased. In well managed domestic catties 8-10 eggs are superovulated; the number may go to 60. However, this depends on health, nutrition, breed of animals and environment in which they live.
Price (1991) has reviewed the current practice of multiple ovulation and superovulation. This technique has spread from cattle and sheep to goats, horses and deers. He has emphasized that (i) general selection for increased litter size is useful only in sheep, (ii) the gonadotropic hormone induces superovulation in goats, sheeps and cattle but the response varies so much, and (iii) immunization against ovarian steroid hormones can increase litter size in sheep. Therefore, different molecular forms of follicle stimulation hormones (FSH) should be characterized in the farm animals. Misra et al. (1990) have reported multiple ovulation and embryo transfer in Indian buffalo (Bubalus bubalis).
After injecting the gonadotrophin the females are induced for superovulation. During follicular phase (second phase of oestrous cycle) about 20 ovarian follicles are induced. Eventually these grow and filled with fluid. The space of follicle which is filled with fluid is called antrum, and such follicles as antral follicles. In normal course, only one follicle develops and releases one egg after maturation. However, before ovulation the follicles laying against surface of ovary looks large sized (8 mm in sheep and pigs, 15 mm in cattle). Therefore, immature oocytes from follicles of donor females are recovered surgically by using laparoscope.
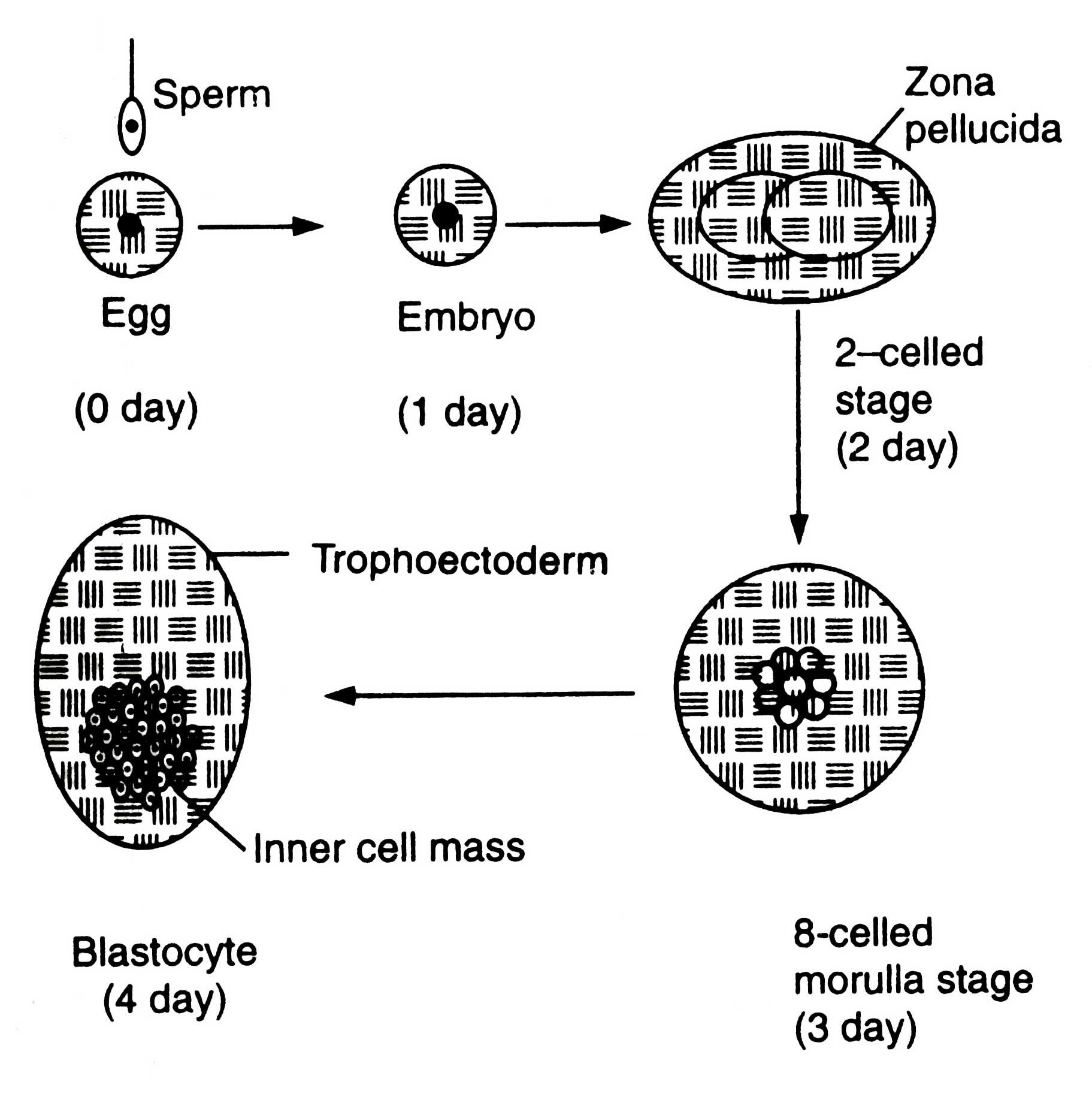
The females to be superovulated are frequently injected with prostaglandin F2a (PGF2a) so that synchronized oestrous could develop in them. After 10 days of oestrous they are injected with hormone FSH up to 4 days followed by PGF2a treatment so that oestrous may be maintained. The FSH treatment induces superovulation. The females are artificially inseminated. The eggs are fertilized. After fertilization embryos undergo developmental stages (Fig. 7.1).
Multiple Ovulation with Embryo Transfer (MOET)
After 6-8 days of fertilization (for sheep and goat) the embryos are recovered. During this stage embryos have come to morula or blastula atage and remain in female's oviduct. In cattle the embryos are recovered without surgery by inserting a catheter into oviduct. A saline solution is drained in oviduct and embryos flushed out through catheter into a storage bottle. The oviduct of sheep is small, therefore, it is exposed surgically and embryos are recovered by syringe adopting the same method for cattle.
In storage bottle embryos settle down and solution decanted. Embryos with small volume of saline are poured into a Petri dish. Then they are examined under microscope. The identified embryos are transferred into synchronized recipient females (|as artificial insemination), when they are in 6-8 days of cycle of embryo development, stored and transported or manipulated as desired. In general, one superovulated female results into 5-6 progenies. In cattle 50-60 per cent of pregnancy can be achieved from embryo transfer method (Ebert, 1989).
Embryo splitting (Demi-embryo)
Embryo of blastocyst stage (Fig. 7.2) is differentiated into two regions, trophoectoderm and inner cell mass (ICM).
Trophoectoderm is a single layer of trophoblast cells lining the inner side of zone surrounding the blastoceal (a fluid filled cavity). Zona becomes the foetal membrane bf placenta. The ICM is the mass of cells which develops into foetus.
The embryos of blastocyst stage can be split into equal two halves and transferred to females to produce identical twins. Thus, embryo splitting technology has increased the rate of pregnancy. To carry out embryo splitting, the blastocyst stage embryos are transferred for a few minutes into a cell culture medium consisting of hypertonic sucrose and bovine serum albumin.
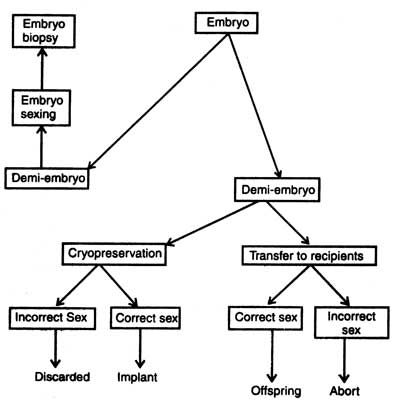
The medium of high osmotic strength enters into cell membrane of the embryo (zona pellucida) and cells contract due to exosmosis. The bovine albumin serum attaches to zona pellucida and provides negative charge to the embryo. Then it is transferred into a plastic Petri dish containing standard cell culture medium. The outer membrane of embryo is negatively charged and Petri dish positively. Embryo sticks to the surface of Petri dish due to development of electrostatic interaction of two charges. The Petri dish is kept on stage of an inverted microscope. A micromanipulator equipped with fine surgical blade is used to cut the embryo roughly into two halves with minimal damage to the cells. The hemispherical mass of half embryo, demi-embryo or semi-embryo reforms spheres.
However, it is not necessary for successful implantation of demi-embryo that it should be enclosed in a zona pellucida. The demi-embryos are transferred into oviduct of synchronized recipients as described for normal embryo transfer. At this stage it is very necessary to know the situation for successful embryo implantation that the wall of uterus of synchronized female recipient is waiting for embryo, and embryo is prepared to send chemical signals from the trophoblast (Ebert, 1989).
To increase the rate of pregnancy, the embryo can be cut into equal three or four pieces, and transplanted in synchronized females. But too small trophoblast will not induce the uterus for pregnancy. So far it is unknown how cells of ICM are required for successful pregnancy, but it is clear that increasing in embryo splitting will have less probability of pregnancy.
Embryo biopsy (removal of small number of cells for genetic analysis) should be combined with splitting so that the twins which will be produced should be identical and of known genotype. Biopsy is very necessary in breeding so that sex and genetic diseases could be detected. In case the embryo has any genetic disease, it can be prevented from implantation in recipient females.
Embryo sexing
Before the implantation of embryo its sex should be detected from the biopsy sample. The principle for sexing is very common. The presence of Y chromosome makes the offsprings male and that of X makes female. The second method is the use of polymerase chain reaction ( PCR) machine in sex detection. PCR amplifies DNA sequence of Y chromosome and reaction products can be seen directly. Handy side et al. (1989) isolated single blastomere from early embryo from a womb, amplified DNA sequences of Y chromosome and carried out embryo sexing before implantation into uterus. It is true that the PCR was first commercially implemented for embryo sexing of livestock (for detail description see Genes : Nature, Concept and Synthesis. Now the sexed embryos are available commercially but these are very costly.
Secondly, ruminant female carries one pregnancy at a time as only one egg is produced and fertilized with male's sperm. Thus, there is a chance for increasing the number of egg production at a time and transfer of fertilized eggs i.e. embryo into uterus of less important foster mothers other than original female in farm animal.
The reproductive cycle of ruminant female is such that the ovarian follicle of a non-pregnant female matures and releases single egg at a time. The time of ovulation differs in different animals, for example 21 days in cows and horse, 16 days in sheep and goats, etc. Normally ovulation occurs as a result of circulation of gonadotropic hormone. But by increasing the concentration of hormone the number of egg production gets increased. In well managed domestic catties 8-10 eggs are superovulated; the number may go to 60. However, this depends on health, nutrition, breed of animals and environment in which they live.
Price (1991) has reviewed the current practice of multiple ovulation and superovulation. This technique has spread from cattle and sheep to goats, horses and deers. He has emphasized that (i) general selection for increased litter size is useful only in sheep, (ii) the gonadotropic hormone induces superovulation in goats, sheeps and cattle but the response varies so much, and (iii) immunization against ovarian steroid hormones can increase litter size in sheep. Therefore, different molecular forms of follicle stimulation hormones (FSH) should be characterized in the farm animals. Misra et al. (1990) have reported multiple ovulation and embryo transfer in Indian buffalo (Bubalus bubalis).
The females to be superovulated are frequently injected with prostaglandin F2a (PGF2a) so that synchronized oestrous could develop in them. After 10 days of oestrous they are injected with hormone FSH up to 4 days followed by PGF2a treatment so that oestrous may be maintained. The FSH treatment induces superovulation. The females are artificially inseminated. The eggs are fertilized. After fertilization embryos undergo developmental stages (Fig. 7.1).
After 6-8 days of fertilization (for sheep and goat) the embryos are recovered. During this stage embryos have come to morula or blastula atage and remain in female's oviduct. In cattle the embryos are recovered without surgery by inserting a catheter into oviduct. A saline solution is drained in oviduct and embryos flushed out through catheter into a storage bottle. The oviduct of sheep is small, therefore, it is exposed surgically and embryos are recovered by syringe adopting the same method for cattle.
In storage bottle embryos settle down and solution decanted. Embryos with small volume of saline are poured into a Petri dish. Then they are examined under microscope. The identified embryos are transferred into synchronized recipient females (|as artificial insemination), when they are in 6-8 days of cycle of embryo development, stored and transported or manipulated as desired. In general, one superovulated female results into 5-6 progenies. In cattle 50-60 per cent of pregnancy can be achieved from embryo transfer method (Ebert, 1989).
Embryo splitting (Demi-embryo)
Embryo of blastocyst stage (Fig. 7.2) is differentiated into two regions, trophoectoderm and inner cell mass (ICM).
Trophoectoderm is a single layer of trophoblast cells lining the inner side of zone surrounding the blastoceal (a fluid filled cavity). Zona becomes the foetal membrane bf placenta. The ICM is the mass of cells which develops into foetus.
The embryos of blastocyst stage can be split into equal two halves and transferred to females to produce identical twins. Thus, embryo splitting technology has increased the rate of pregnancy. To carry out embryo splitting, the blastocyst stage embryos are transferred for a few minutes into a cell culture medium consisting of hypertonic sucrose and bovine serum albumin.
However, it is not necessary for successful implantation of demi-embryo that it should be enclosed in a zona pellucida. The demi-embryos are transferred into oviduct of synchronized recipients as described for normal embryo transfer. At this stage it is very necessary to know the situation for successful embryo implantation that the wall of uterus of synchronized female recipient is waiting for embryo, and embryo is prepared to send chemical signals from the trophoblast (Ebert, 1989).
To increase the rate of pregnancy, the embryo can be cut into equal three or four pieces, and transplanted in synchronized females. But too small trophoblast will not induce the uterus for pregnancy. So far it is unknown how cells of ICM are required for successful pregnancy, but it is clear that increasing in embryo splitting will have less probability of pregnancy.
Embryo sexing
Before the implantation of embryo its sex should be detected from the biopsy sample. The principle for sexing is very common. The presence of Y chromosome makes the offsprings male and that of X makes female. The second method is the use of polymerase chain reaction ( PCR) machine in sex detection. PCR amplifies DNA sequence of Y chromosome and reaction products can be seen directly. Handy side et al. (1989) isolated single blastomere from early embryo from a womb, amplified DNA sequences of Y chromosome and carried out embryo sexing before implantation into uterus. It is true that the PCR was first commercially implemented for embryo sexing of livestock (for detail description see Genes : Nature, Concept and Synthesis. Now the sexed embryos are available commercially but these are very costly.