Light Dependent Reactions
Photosynthetic light reactions take place in thylakoid membranes where chromophore–protein complexes and membrane-bound enzymes are situated. The thylakoid membrane cannot be considered as a rigid, immutable structure. It is rather a highly dynamic system, the molecular compositions and conformation of which, including the spatial pattern of its components, can change very
rapidly. This flexibility, is, however, combined with a high degree of order necessary for the energy-transforming processes.
Quantitative analysis established that the 7 nm thick thylakoid membrane consists of approximately 50% lipids and 50% proteins. Galactolipids, a constituent that is specific of thylakoid membranes, make up approximately 40% of the lipid fraction. Chlorophylls
a, b, c1 and
c2, phospholipids, sulfolipids, carotenoids, xanthophylls, quinones, and sterols, all components occurring in a bound form, represent the remainder 10%.
Chlorophyll a consists of a hydrophilic porphyrin head formed by four linked pyrrole rings with a magnesium atom chelated (Mg
2+) at the center and a hydrophobic phytol tail. Chlorophyll
b possesses the same structure as chlorophyll
a but
a keto group (-CH=O) is present in the second pyrrole ring instead of a methyl group (-CH
3). Chlorophyll c possesses only the hydrophilic porphyrin head without the phytol tail; chlorophyll
c2 differs from chlorophyll
c1 by possessing two vinyl groups (-CH=CH
2) instead of one. In the phycobiliproteins the four pyrrolic rings are linearly arranged, and unlike the chlorophylls they are strongly covalently bound to a protein. Carotenoids are C40 hydrocarbon chains, strongly hydrophobic, with one or two terminal ionone rings. The xanthophylls are carotenoid derivates with a hydroxyl group in the ring (Figure 3.2).
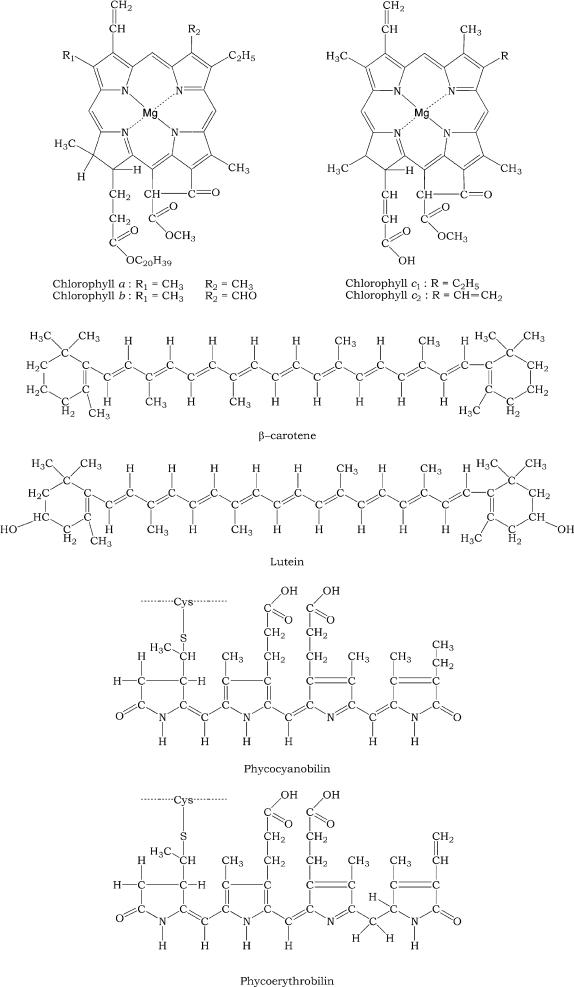
FIGURE 3.2 Structure of the main pigments of the thylakoid membrane.
The protein complex content consists mainly of the highly organized energy transforming units, enzymes for the electron transport, and ATP-synthesis, more or less integrated into the thylakoid membrane. The energy transforming units are two large protein complexes termed photosystems I (PSI) and II (PSII), surrounded by light harvesting complexes (LHCs). Photons absorbed by PSI and PSII induce excitation of special chlorophylls, P
700 and P
680 (P stands for pigment and 700/680 stand for the wavelength in nanometer of maximal absorption), initiating translocation of an electron across the thylakoid membrane along organic and inorganic redox couples forming the electron transfer chains (ETCs). The main components of these chains are plastoquinones, cytochromes, and ferredoxin. This electron translocation process eventually leads to a reduction of NADP
+ to NADPH and to a transmembrane difference in the electrical potential and H
+ concentration, which drives ATP-synthesis by means of an ATP-synthase.
Thylakoid membranes are differentiated into stacked and unstacked regions. Stacking increases the amount of thylakoid membrane in a given volume. Both regions surround a common internal thylakoid space, but only unstacked regions make direct contact with the chloroplast stroma. The two regions differ in their content of photosynthetic assemblies; PSI and ATP-synthase are located almost exclusively in unstacked regions, whereas PSII and LCHII are present mostly in stacked regions. This topology derived from protein–protein interactions rather than lipid bilayers interactions. A common internal thylakoid space enables protons liberated by PSII in stacked membranes to be utilized by ATP-synthase molecules that are located far away in unstacked membranes. What is the functional significance of this lateral differentiation of the thylakoid membrane system? If both photosystems were present at high density in the same membrane region, a high proportion of photons absorbed by PSII would be transferred to PSI because the energy level of the excited state P*
680 relative to its ground state P
680 is higher than that of P*
700 relative to P
700.
A lateral separation of photosystems solves this problem by placing P*
680 more than 100 Å away from P
700. The positioning of PSI in the unstacked membranes gives it a direct access to the stroma for the reduction of NADP
+. In fact the stroma-exposed surface of PSI, which contains the iron-sulfur proteins that carry electron to ferredoxin and ultimately to NADP
+, protrudes about 50 Å beyond the membrane surface and could not possibly be accommodated within the stacks, where adjacent thylakoids are separated by no more than 40 Å . It seems likely that ATP-synthase is also located in unstacked regions to provide space for its large protruding portion and access to ADP. In contrast, the tight quarters of the appressed regions do not pose a problem for PSII, which interacts with a small polar electron donor (H
2O) and a highly lipid-soluble electron carrier (plastoquinone). According to the model of Allen and Forsberg (2001), the close
appression of grana (stacks of thylakoids) membranes arises because the flat stroma-exposed surfaces of LHCII form recognition and contact surfaces for each other, causing opposing surfaces of thylakoids to interact. There is not steric hindrance to this close opposition of stacked grana membranes, because similar to LHCII PSII presents a flat surface that protrudes not more than 10–20 Å beyond the membrane surface.
The functional significance of thylakoid stacking is presumably to allow a large, connected, light-harvesting antenna to form both within and between membranes. Within this antenna both the excitation energies can pass between chlorophylls located in LHCII complexes that are adjacent to each other, both within a single membrane and between appressed membranes.
The degree of stacking and the proportion of different photosynthetic assemblies are regulated in response to environmental variables such as the intensity and spectral characters of incident light. The lateral distribution of LHC is controlled by reversible phosphorylation. At low light levels, LHC is bound to PSII. At high light levels, a specific kinase is activated by plastoquinol, and phosphorylation of threonine side chains of LHC leads to its release from PSII. The phosphorylated form of these light harvesting units diffused freely in the thylakoid membrane and may become associated with PSI to increase its absorbance coefficient (Figure 3.3).
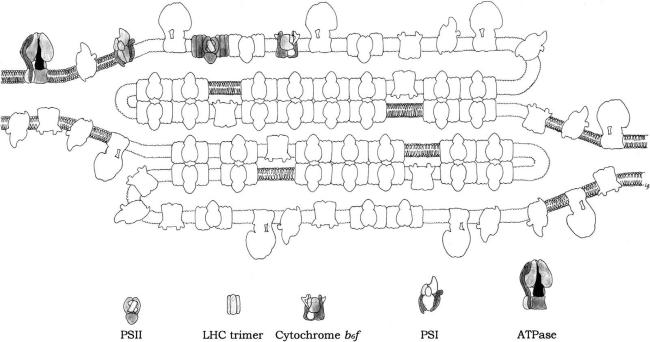
FIGURE 3.3 Model for the topology of chloroplast thylakoid membrane, and for the disposition within the chloroplast of the major intrinsic protein complexes, PSI, PSII, LHCII trimer, Cytochrome b6f dimer and ATPase. (Redrawn after Allen and Forsberg, 2001.)
Central to the photosynthetic process is PSII, which catalyzes one of the most thermodynamically demanding reactions in biology: the photo-induced oxidation of water (2H
2O → 4e
- + 4H
+ + O
2). PSII has the power to split water and use its electrons and protons to drive photosynthesis. The first ancestor bacteria carrying on anoxygenic photosynthesis probably synthesized ATP by oxidation of H
2S and FeS compounds, abundant in the environment. The released energy could have been harnessed via production of a proton gradient, stimulating evolution of electron transport chains, and the reducing equivalents (electrons) generated used in CO
2 fixation and hence biosynthesis. This was the precursor of the PSI. About 2800 million years ago the evolutionary pressure to use less strongly reducing (and therefore more abundant) source of electrons appears to have culminated in the development of the singularly useful trick of supplying the electrons to the oxidized reaction center from a tyrosine side chain, generating tyrosine cation radicals that are capable of sequential abstraction of electrons from water.
Oxygenic photosynthesis, which requires coupling in series of two distinct types of reaction centers (PSI and PSII) must have depended on later transfer of genes between the evolutionary precursors of the modern sulfur bacteria (whose single reaction center resembles PSI) and those of purple bacteria (whose single reaction center resembles PSII). Thus the cyanobacteria appeared. They were the first dominant organisms to use photosynthesis. As a by-product of photosynthesis, oxygen gas (O
2) was produced for the first time in abundance. Initially, oxygen released by photosynthesis was absorbed by iron(II), then abundant in the sea, thus oxidizing it to insoluble iron(III) oxide (rust). Red “banded iron deposits” of iron(III) oxide are marked in marine sediments of ca. 2500 million years ago. Once most/all iron(II) had been oxidized to iron(III), then oxygen appeared in,
and began to increase in the atmosphere, gradually building up from zero ca. 2500 million years ago to approximately present levels ca. 500 million years ago. This was the “oxygen revolution.” Oxygen is corrosive, so prokaryotic life then either became extinct, survived in anaerobic (oxygen free) environments (and do so to this day), or evolved antioxidant protective mechanisms.
The latter could begin to use oxygen to pull electrons from organic molecules, leading to aerobic respiration. The respiratory ETC probably evolved from established photosynthetic electron transport, and the citric acid cycle probably evolved using steps from several biosynthetic pathways.
Hence cyanobacteria marked the planet in a very permanent way and paved the way for the subsequent evolution of oxidative respiratory biochemistry. This change marks the end of the Archaean Era of the Precambrian Time.






