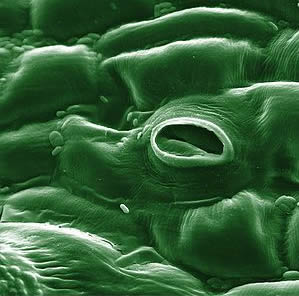
Stoma
In botany, a stoma (also stomate; plural stomata) is a pore, found in the leaf and stem epidermis that is used for gas exchange. The pore is formed by a pair of specialized parenchyma cells known as guard cells which are responsible for regulating the size of the opening. Air containing carbon dioxide enters the plant through these openings where it is used in photosynthesis and respiration. Oxygen produced by photosynthesis in the spongy layer cells (parenchyma cells with pectin) of the leaf interior exits through these same openings. Also, water vapor is released into the atmosphere through these pores in a process called transpiration.Stomata are present in the sporophyte generation of all land plant groups except liverworts. Dicotyledons usually have more stomata on the lower epidermis than the upper epidermis. Monocotyledons, on the other hand, usually have the same number of stomata on the two epidermes. In plants with floating leaves, stomata may be found only on the upper epidermis; submerged leaves may lack stomata entirely.
The word stoma derives from Greek στόμα 'mouth'.
Contents
» Function» Carbon gain and water loss
» Alternative approaches
» CAM plants
» Opening and closure
» Inferring stomatal behavior from gas exchange
» Evolution
» Development
» Stomata as pathogenic pathways
» References
Function
Carbon gain and water loss
Carbon dioxide, a key reactant in photosynthesis, is present in the atmosphere at a concentration of about 384 ppm (as of March 2008). Most plants require the stomata to be open during daytime. The problem is that the air spaces in the leaf are saturated with water vapor, which exits the leaf through the stomata (this is known as transpiration). Therefore, plants cannot gain carbon dioxide without simultaneously losing water vapor.Alternative approaches
Ordinarily, carbon dioxide is fixed to ribulose-1,5-bisphosphate (RuBP) by the enzyme RuBisCO in mesophyll cells exposed directly to the air spaces inside the leaf. This exacerbates the carbon/water tradeoff for two reasons: first, Rubisco has a relatively low affinity for carbon dioxide and second, it fixes oxygen to RuBP, wasting energy and carbon in a process called photorespiration.For both of these reasons, Rubisco needs high carbon dioxide concentrations, which means high stomatal apertures and consequently high water loss.
However, plants possess another enzyme that can also fix carbon dioxide: PEP carboxylase or PEPCase. This enzyme has high carbon dioxide affinity, so a given rate of carbon dioxide fixation can be achieved with less stomatal opening, and hence less water loss. The catch is that the products of carbon fixation by PEPCase must be converted in an energy-intensive process to continue through the carbon reactions of photosynthesis. As a result, the PEPCase alternative is only preferable where water is more limiting but light — which provides the energy in this case — is plentiful, and/or where high temperatures increase the solubility of oxygen relative to that of carbon dioxide, magnifying Rubisco's oxygenation problem.
CAM plants
A group of mostly desert plants called "CAM" plants (Crassulacean acid metabolism, after the family Crassulaceae, which includes the species in which the CAM process was first discovered) open their stomata at night (when water evaporates more slowly from leaves for a given degree of stomatal opening), use PEPcarboxylase to fix carbon dioxide and store the products in large vacuoles. The following day, they close their stomata and release the carbon dioxide fixed the previous night into the presence of RuBisCO. This saturates RuBisCO with carbon dioxide, allowing minimal photorespiration. This approach, however, is severely limited by the capacity to store fixed carbon in the vacuoles, so it is preferable only when water is severely limiting.Opening and closure
However, most plants do not have the aforementioned facility and must therefore open and close their stomata during the daytime in response to changing conditions, such as light intensity, humidity, and carbon dioxide concentration. It is not entirely certain how these responses work. However, the basic mechanism involves regulation of osmotic pressure.When conditions are conducive to stomatal opening (e.g., high light intensity and high humidity), a proton pump drives protons (H+) from the guard cells. This means that the cells' electrical potential becomes increasingly negative. The negative potential opens potassium voltage - gated channels and so an uptake of potassium ions (K+) occurs. To maintain this internal negative voltage so that entry of potassium ions does not stop, negative ions balance the influx of potassium. in some cases chloride ions enter, while in other plants the organic ion malate is produced in guard cells. This in turn increases the osmotic pressure inside the cell, drawing in water through osmosis. This increases the cell's volume and turgor pressure. Then, because of rings of cellulose microfibrils that prevent the width of the guard cells from swelling, and thus only allow the extra turgor pressure to elongate the guard cells, whose ends are held firmly in place by surrounding epidermal cells, the two guard cells lengthen by bowing apart from one another, creating an open pore through which gas can move.
Confocal microscopy image of an Arabidopsis thaliana stoma showing two guard cells exhibiting fluorescence from green fluorescent protein and native chlorophyll (red)
Interestingly, guard cells have more chloroplasts than the other epidermal cells from which guard cells are derived. Their functi on is controversial.
Inferring stomatal behavior from gas exchange
Another way to find out whether stomata are open or closed, or more accurately, how open they are, is by measuring leaf gas exchange. A leaf is enclosed in a sealed chamber and air is driven through the chamber. By measuring the concentrations of carbon dioxide and water vapor in the air before and after it flows through the chamber, one can calculate the rate of carbon gain (photosynthesis) and water loss (transpiration) by the leaf.However, because water loss occurs by diffusion, the transpiration rate depends on two things: the gradient in humidity from the leaf's internal air spaces to the outside air, and the diffusion resistance provided by the stomatal pores. Stomatal resistance (or its inverse, stomatal conductance) can therefore be calculated from the transpiration rate and humidity gradient. (The humidity gradient is the humidity inside the leaf, determined from leaf temperature based on the assumption that the leaf's air spaces are saturated with vapor, minus the humidity of the ambient air, which is measured directly.) This allows scientists to learn how stomata respond to changes in environmental conditions, such as light intensity and concentrations of gases such as water vapor, carbon dioxide, and ozone.
Evolution
The fossil record has little to say about the evolution of stomata. They may have evolved by the modification of conceptacles from plants' alga-like ancestors.Development
There are three major epidermal cell types which all ultimately derive from the L1 tissue layer of the shoot apical meristem, called protodermal cells: trichomes, pavement cells and guard cells, all of which are arranged in a nonrandom fashion. An asymmetrical cell division occurs in protodermal cells resulting in one large cell that is fated to become a pavement cell and a smaller cell called a meristemoid that will eventually differentiate into the guard cells that surround a stoma. This meristemoid then divides asymmetrically one to three times before differentiating into a guard mother cell. The guard mother cell then makes one symmetrical division, which forms a pair of guard cells.Stomata as pathogenic pathways
Stomata are an obvious hole in the leaf by which, as was presumed for a while, pathogens can enter unchallenged. However, it has been recently shown that stomata do in fact sense the presence of some, if not all, pathogens. However, with the virulent bacteria applied to Arabidopsis plant leaves in the experiment, the bacteria released the chemical coronatine, which forced the stomata open again within a few hours.References
- N. S. CHRISTODOULAKIS; J. MENTI and B. GALATIS (January 2002). "Structure and Development of Stomata on the Primary Root of Ceratonia siliqua L.". Annals of Botany 89 (1): 23–29.- C. L. Trejo; W. J. Davies; LdMP. Ruiz (1993). "Sensitivity of Stomata to Abscisic Acid (An Effect of the Mesophyll)". Plant Physiology 102 (2): 497–502.
- Petra Dietrich; Dale Sanders; Rainer Hedrich (October 2001). "The role of ion channels in light-dependent stomatal opening". Journal of Experimental Botany 52 (363): 1959–1967.
- Eduardo Zeiger; Lawrence D. Talbott; Silvia Frechilla; Alaka Srivastava; Jianxin Zhu (March 2002). "The Guard Cell Chloroplast: A Perspective for the Twenty-First Century". New Phytologist 153 (3 Special Issue: Stomata): 415–424.
- D. Edwards, H. Kerp; Hass, H. (1998), "Stomata in early land plants: an anatomical and ecophysiological approach", Journal of Experimental Botany 49: 255–278.
- Krassilov, Valentin A. (2004). "Macroevolutionary events and the origin of higher taxa". in Wasser, Solomon P.. Evolutionary theory and processes : modern horizons : papers in honour of Eviatar Nevo. Dordrecht: Kluwer Acad. Publ.. pp. 265-289.
- Bergmann, Dominique C.; Lukowitz, Wolfgang; Somerville, Chris R. (4 July 2004), "Stomatal Development and Pattern Controlled by a MAPKK Kinase", Science 304: 1494–1497.
- Maeli Melotto; William Underwood, Jessica Koczan, Kinya Nomura, Sheng Yang He (September 2006). "Plant Stomata Function in Innate Immunity against Bacterial Invasion". Cell 126: 969–980.