Functions in Plants
The ability of iron to undergo a valence change is important in its functions:It is also the case that iron easily forms complexes with various ligands, and by this modulates its redox potential. Iron is a component of two major groups of proteins. These are the heme proteins and the Fe-S proteins. In these macromolecules, the redox potential of the Fe(III)/Fe(II) couple, normally 770mV, can vary across most of the range of redox potential in respiratory and photosynthetic electron transport (-340 to +810 mV). When iron is incorporated into these proteins it acquires its essential function (9).
The heme proteins contain a characteristic heme iron–porphyrin complex, and this acts as a prosthetic group of the cytochromes. These are electron acceptors–donors in respiratory reactions. Other heme proteins include catalase, peroxidase, and leghemoglobin.
Catalase catalyzes the conversion of hydrogen peroxide into water and O2 (reaction A), whereas peroxidases catalyze the conversion of hydrogen peroxide to water (reaction B):
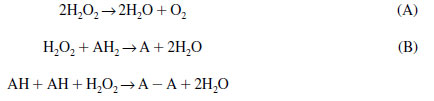 |
Catalase has a major role in the photorespiration reactions, as well as in the glycolate pathway, and is involved in the protection of chloroplasts from free radicals produced during the water-splitting reaction of photosynthesis. The reaction sequence of peroxidase shown above includes cell wall peroxidases, which catalyze the polymerization of phenols to form lignin. Peroxidase activity is noticeably depressed in roots of iron-deficient plants, and inhibited cell wall formation and lignification, and accumulation of phenolic compounds have been reported in iron-deficient roots.
As well as being a constituent of the heme group, iron is required at two other stages in its manufacture. It activates the enzymes aminolevulinic acid synthetase and coproporphorinogen oxidase. The protoporphyrin synthesized as a precursor of heme is also a precursor of chlorophyll, and although iron is not a constituent of chlorophyll this requirement, and the fact that it is also required for the conversion of Mg protoporphyrin to protochlorophyllide, means that it is essential for chlorophyll biosynthesis (10). However, the decreased chloroplast volume and protein content per chloroplast (11) indicate that chlorophyll might not be adequately stabilized as chromoprotein in chloroplasts under iron deficiency conditions, thus resulting in chlorosis.
Along with the iron requirement in some heme enzymes and its involvement in the manufacture of heme groups in general, iron has a function in Fe-S proteins, which have a strong involvement with the light-dependent reactions of photosynthesis. Ferredoxin, the end product of photosystem I, has a high negative redox potential that enables it to transfer electrons to a number of acceptors. As well as being the electron donor for the synthesis of NADPH in photosystem I, it can reduce nitrite in the reaction catalyzed by nitrite reductase and it is an electron donor for sulfite reductase.




