Diffusion
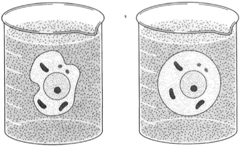 |
| Figure 8-4 Osmosis. Water flows toward a region of greater concentration of dissolved substance. A cell placed in pure water soon becomes turgid by the inflow of water, as shown at right. |
Consider some substances in true solution. Substances tend to diffuse, that is, move from a region where they are in greater concentration to a region where they are in lesser concentration. This is simply because molecules are in constant motion. If a cell membrane is involved, water can pass readily through but dissolved material may or may not be able to pass through. Whether or not dissolved material is able to pass through a cell membrane depends in part on the size of the molecules. Remember, a cell membrane is a selectively permeable, or osmotic, membrane. The process of water passing through the membrane is called osmosis. Water will tend to flow from a region of greater concentration of water to a region of lesser concentration of water. Another and perhaps more common way of saying this is that water tends to flow toward a greater concentration of dissolved substances.
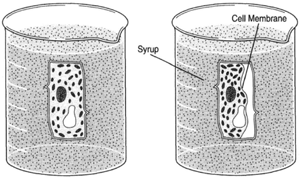 |
| Figure 8-5 The direction of water flow is reversed when cells are placed in syrup, water tending to flow toward a region of greater concentration of dissolved substance. The process is the same, only the direction is changed. |
When cells are placed in pure water, there being a dissolved substance inside the cells, water tends to flow into the cells, producing turgor. The resulting condition of turgor pressure then tends to cause water to flow out of the cell. Soon, equilibrium is attained. The flow of water, then, does not stop; rather, there is an equal rate of flow in both directions. The pressure that builds up in the cell can sometimes cause the cell to burst, or lyse. In some plant cells, this is prevented by the presence of rigid cell walls.
When cells are placed in a concentrated solution such as syrup, the concentration of dissolved substances (sugar) is greater outside the cells, and water tends to flow out of the cells, causing the cells to shrink.
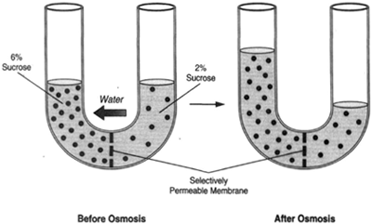 |
| Figure 8-6 A U-tube with an osmotic membrane centered at the bottom. Water flows toward a greater concentration of sugar. |
A solution having a lesser concentration of dissolved material as is present in the cells is called a hypotonic solution; a solution having the same concentration of dissolved material as is present in the cells is called an isotonic solution; and a solution having a greater concentration of dissolved material than is present in the cells is called a hypertonic solution.
Plant cells having rigid cell walls are good material for demonstrating diffusion, turgor, and differences between solutions. Such cells placed in pure water (a hypotonic solution) become turgid, the cell membranes pressing against the cell walls. The cells do not lyse, and the process is reversible. Such cells placed in a hypertonic solution will shrink, water flowing out of the cells and thus causing the cell membranes to pull away from the cell walls. This process, called plasmolysis, can be reversed.
Figure 8-6 shows a U-tube. An osmotic membrane at the bottom of the tube forms a partition between the two sides. A 6-percent solution of sucrose is placed on one side, and a 2-percent solution of sucrose is placed on the other side. Water, tending to flow toward a greater concentration of dissolved substance, will flow from the 2-percent solution to the 6-percent solution, causing the level of the liquid on the left side to rise. This, in turn, causes a
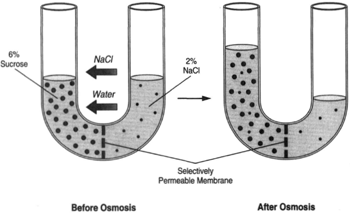 |
| Figure 8-7 A U-tube with a sodium chloride solution on the right side of the tube. The Same tendencies would occur causing the volume on the right side of the tube to diminish. |
Figure 8-7 shows a U-tube with a 6-percent solution of sucrose on the left side and a 2-percent solution of sodium chloride on the right side. Again, water wil flow toward a greater concentration of dissolved substance (toward the sugar). Where as the selectively permeable membrane does not allow the sugar. to pass through, the sodium chloride is readily able to pass through. This causes the concentration of sodium chloride to become the same on both sides.
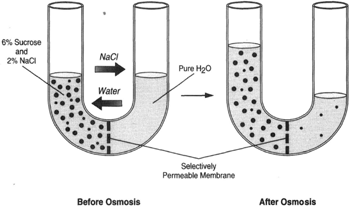 |
Figure 8-8 If both sugar and sodium chloride are placed in the same side of the tube, the rules are the same; a dissolved substance tends to flow toward a region of lesser concentration of that substance. This applies to both the sugar and the salt. But whereas the sugar confronts a barrier in the osmotic membrane, the osmotic membrane does not present a barrier to the sodium chloride. In time, the concentration of sodium chloride will be the same on both sides of the membrane. The sugar, however, will remain on the left. |
Figure 8-8 shows a U-tube with a 6-percent solution of sucrose and a 2-percent solution of sodium chloride (salt) on the left side, and pure water on the right side. In time, the salt concentration will equalize on both sides. Whereas osmosis is the movement of water, dialysis is the movement of salt.
In the U-tube examples, the force that allows the movement of water and solutes is diffusion, which is itself caused by the molecules being in constant motion. In living systems, the same force is at work; but other factors also come into play. Membranes, being alive, are able to exert additional forces. For example, solutions can be moved against a concentration gradient, that is, in a direction opposite that previously described. Such movement requires the expenditure of energy and is called active transport. The large, brown seaweeds offer an example of active transport. These organisms are able to concentrate iodine. Iodine is present in extremely minute quantities in sea water, but it is taken in through the living membranes and concentrated in these seaweeds. For a long time these seaweeds, called kelp, were used as a commercial source of iodine. Certain kinds of plants are able to concentrate potassium. There may be a 50 to even 1,000 times greater concentration of potassium in the cells of such plants than exists in the surrounding soil. Certain fungi concentrate metals. All such examples of active transport require the expenditure of energy.




