Interferons were once believed to have the ability to fight cancer and, therefore, received major attention of molecular biologists, even though its early clinical promise was not fulfilled. However, recent studies with interferon gave information about how cells really respond to interferon signals. In the year, 1992, several teams of researchers demonstrated that when interferon contacts its receptor on the cell surface, it immediately activates a transcription factor which moves to the nucleus, where it turns on its own particular set of genes. It was experimentally demonstrated that no
'second messengers' are involved in this signal transduction pathway.
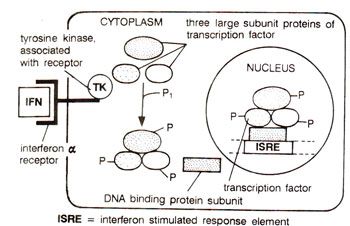
Fig. 37.26. A direct route for interferon induced signal transductibn pathway, where the tyrosine kinase (TK) associated with the interferon a-receptor complex activates the transcription factor directly, thus turning on the interferon responsive genes.
In mid-1980s, it was shown that the genes that respond to interferon carry a common regulator sequence called
ISRE (interferon stimulated response element). In late 1980s, the transcription factor that binds to ISRE was also discovered. This transcription factor consists of four protein subunits including three large subunits, and a small subunit. The fourth small subunit actually makes contact with the ISRE. It was also shown that the three large subunits of the transcription factor, each contains an SH2 domain, a sequence commonly found in proteins that interact with tyrosine kinase.
In cells, which have not been stimulated by interferon-a, the three large subunits of the above transcription factor, are not linked and are located only in the cytoplasm. But once interferon-α binds to its receptor, these three subunits rapidly undergo phosphorylation at the tyrosine residues due to
tyrosine kinase associated with the receptor. The phosphorylated protein subunits associate together and move to the nucleus, where they join the smaller subunit to make theactive transcription factor, thus conferring on it the ability to bind on ISRE. This leads to interferon-α stimulated expression of specific genes (Fig. 37.26).

Fig. 37.26. A direct route for interferon induced signal transductibn pathway, where the tyrosine kinase (TK) associated with the interferon a-receptor complex activates the transcription factor directly, thus turning on the interferon responsive genes.





