Uses of monoclonal antibodies
Monoclonal antibodies or specific antibodies, are now an essential tool of much biomedical research and are of great commercial and medical value. For instance, ABO blood groups could be earlier identified with the help of human sera carrying antibodies of known specificity. These human sera in U.K. have been replaced by monoclonal antibodies produced by hybridomas, for the identification of ABO blood groups. Thus the diagnostic and screening value of the monoclonal antibodies through serological tests has been demonstrated. Besides the use of monoclonals in identification of blood groups in UK (UK blood typing), following four uses for monoclonais are described, although, only the first two of these make a definite market at present : (i) diagnosis (including ELISA test for detection of viruses); (ii) immunopurification; (iii) imaging and (iv) therapy.
Antibodies have also been used to immunize humans and cattle against certain diseases. The most promising outcome in this area is the prospect of developing antimalarial vaccine in the near future. Monoclonal antibodies that inhibit the in vitro multiplication of Plasmodium, and the antigametocyte antibodies that inactivate male gametes have been developed. Monoclonal antibodies that destroy merozoite infected red blood cells have also been developed now. Such antibodies may prove useful as vaccines.
Monoclonal antibodies as enzymes (abzymes)
Monoclonal antibodies are also used as enzymes that are described as abzymes. The antibodies may often bind specific ligands (haptens), but may not carry out chemical reactions. By modifying these ligands, antibodies may be generated that will catalyse specific reactions just like enzymes. Production of these abzymes is based on the following two principles : (i) enzymes work by binding the transition state of a reactant better than the ground state; (ii) antibodies which bind to specific small molecules can be produced by coupling this small molecule to a protein carrier and using this protein for immunizing experimental animals. If this small molecule is a transition state analogue (molecules that will mimic the shape and electronic configuration of transition state), then the antibodies that were produced to bind to this molecule will function as enzyme towards the substrate of this reaction. Considerable success in the production of abzymes catalyzing following reactions has been achieved : (i) acyl transfer reaction, (ii) carbon-carbon bond formation, (iii) carbon-carbon bond cleavage. A number of monoclonal antibodies using the above approach have been produced to be used as abzymes. Several of these antibodies were shown to accelerate the reactions, which they were supposed to catalyse.
Antibodies have been used in the purification and quantitation of certain molecules present in trace amounts, such as hormones, cyclic nucleotides, polypeptides, enzymes, antigens, etc. The assay is extremely sensitive and quantities as low as nano- or picomolar concentrations can be detected in small volumes (1 ml) of body fluids such as plasma, urine or cerebrospinal fluid. Following steps are involved in the purification and quantitation of enzymes and other proteinaceous molecules.
Raising the antibodies.
Monoclonal antibodies for a specific enzyme are raised using traditional hybridoma technology. For this purpose, enzyme is first extracted and partially purified using most of the conventional steps, such as extraction, ammonium sulfate precipitation and gel/column filtration. The preparation is diluted and emulsified with 'Freunds complete adjuvant'. Then it is injected intramuscularly into mice, to raise antibodies. Generally several doses at intervals of a few days are injected to boost the antibody production. The spleen cells from the mice are collected and fused with myeloma'(tumor) cell lines derived from a rat. The resultant hybridoma cells are screened for their capacity to produce antibodies and secrete them out. The hybridoma cells can be thawed and recultured for the production of antibodies.
Purification and quantitation.
Either the supernatants from cultured hybridoma cells or the ascite fluid is used for detection and purification of an enzyme. Sometimes a specific antibody is purified before it is used for enzyme detection/purification. The most common methods for enzyme purification/detection are : affinity chromatography, radioimmunoassay and immunoprec ipitation.
In affinity chromatography, the monoclonal antibody is generally immobilized on an insoluble matrix such as cross linked dextran or agarose beads, by a covalent linking agent, (e.g. cyanogen bromide). The immobilized antibody is generally packed in a column, where its average concentration is 5-10 mg antibody per ml of bed volume. Protein solution containing the specific enzyme is applied over these immobilized antibodies. The specific enzyme binds with the antibody while other components do not, and thus the specific enzyme is separated. The enzyme can be recovered later by using suitable reagents. Antigens can also be purified using affinity chromatography with monoclonal antibodies. The antigen binds with the immobilized antibody, is eluted and recovered from the immobilized support by suitable washing procedures. Change in pH of the buffer often causes elution. Some important proteins such as alpha fetoprotein and leucocyte interferon are also purified using this procedure.
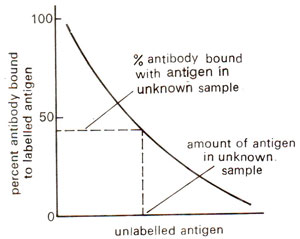
Fig. 43.5. A standard curve used in radioimmunoassay for quantitative estimation of antigen in an unknown sample.
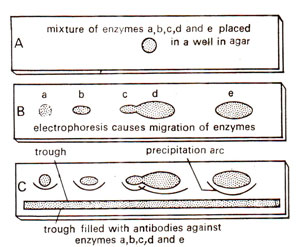
Fig. 43.6. Techniques of immunoprecipitation for detection and separation of enzymes in mixture.
Radioimmunoassay is an extremely sensitive method for the detection and quantitation of any substance that is antigenic and that can be labelled with a radioactive isotope. Basically the method depends upon the competition between labelled (known) and unlabelled (unknown) antigen for the same antibody. A known amount of labelled antigen (enzyme in this case), a known amount of specific antibody and an unknown amount to unlabelled antigen are allowed to reach equilibrium. The amount of labelled antigen bound to the antibody is inversely proportional to the amount of antigen in the unknown sample. By suitably adjusting the amounts of antibody and small amount of antigen in an unknown material can be detected in this way.
In order to measure the amount of labelled antigen attached to antibody, it is necessary to separate the antigen-antibody complexes from the mixture, by measuring the radioactivity, the percentage of labelled antigen bound to the antibody can be calculated. The concentration of antigen in an unknown sample can be determined by reference to a standard curve constructed from data obtained by allowing varying amounts of unlabelled antigen to compete (Fig. 43.5).
When correct conditions are employed, antigen and antibody react to form a precipitate. This precipitation phenomenon has been used to separate and purify enzymes and other antigenic substances in a variety of ways.
One application of immunoprecipitation is immunoelectro-phoresis. This is the most sensitive method for the detection of enzymes or antigens in a mixture. It involves combination of electrophoresis and gel diffusion. In this procedure, the mixture containing the enzyme (or antigen) is placed in a small well, cut in a layer of agar placed on a glass sheet, or a microscopic slide (Fig. 43.6). It is then subjected to electrophoresis by application of an electric current. The enzyme and other components of the mixture migrate through the agar at variable rates. Following electrophoresis, a trough is cut in the gel, parallel to the electrophoretic migration (Fig. 43.6). An antiserum (containing antibody of that specific enzyme) or monoclonal antibody preparation is then placed in the trough. The reactants diffuse towards one another and form separate arcs of precipitate for each antigen-antibody complex. If only one monoclonal antibody preparation is used, then only one precipitation arc is formed.




