Recognition Molecules
Recognition Molecules
We discuss the role of MHC proteins in nonself recognition in the following text, but MHC proteins are not themselves the molecules that recognize foreign substances. This task falls to two basic types of molecules, the genes for which probably evolved from a common ancestor: antibodies and T-cell receptors. Each vertebrate individual has an enormous variety of antibodies, each of which binds specifically to one particular antigen (or part of the antigen), even though that antigen may have never been present in the body previously. There are probably an equal number of different T-cell receptors, each also specific for a particular antigen.
A major problem of immunology is understanding how the mammalian genome could contain the information needed to produce at least a million different antibodies. The answer seems to be that antibody genes occur in pieces, rather than as continuous stretches of DNA, and that the antigenrecognizing sites (variable regions) of the heavy and light chains of the antibody molecules are pieced together from information supplied by separate DNA sequences, which can be shuffled to increase the diversity of the gene products. The immense repertoire of antibodies is achieved in part by complex gene rearrangements and in part by frequent somatic mutations that produce additional variation in protein structure of the variable regions of the heavy and light antibody chains. Analogous processes occur in the production of genes for T-cell receptors.
Antibodies
Antibodies are proteins called immunoglobulins. They are borne in the surface of B lymphocytes or secreted by cells (plasma cells) derived from B cells. The basic antibody molecule consists of four polypeptide strands: two identical light chains and two identical heavy chains held together in a Y-shape by disulfide bonds and hydrogen bonds (Figure 37-2). The amino acid sequence toward the ends of the Y varies in both the heavy and light chains, according to the specific antibody molecule (the variable region), and this variation determines with which antigen the antibody can bind. Each of the ends of the Y forms a cleft that acts as the antigen-binding site (Figure 37-2), and specificity of the molecule depends on the shape of the cleft and properties of the chemical groups that line its walls. The remainder of the antibody is known as the constant region. The variable end of the antibody molecule is often called Fab, for antigen-binding fragment, and the constant end is known as Fc, for crystallizable fragment (Figure 37-2). The so-called constant region is not really constant: the light chains can be either of two types, and the heavy chains may be any of five types. The type of heavy chain determines the class of the antibodies, known as IgM, IgG (now familiar to many people as gamma globulin), IgA, IgD, and IgE. The class of the antibody determines the specific role of the antibody in the immune response (for example, whether the antibody is secreted or held on a cell surface) but not the antigen it recognizes.
Functions of Antibody in Host Defense Antibodies can mediate destruction of an invader (antigen) in a number of ways. A foreign particle, for example, becomes coated with antibody molecules as their Fab regions become bound to it. Macrophages recognize the projecting Fc regions and are stimulated to engulf the particle. This is the process of opsonization. Antibodies also may be able to neutralize toxins that are secreted by an invader.
Another important process, particularly in destruction of bacterial cells, is interaction with complement activated by the classical pathway. As noted previously, the first component in the classical pathway is activated by bound antibody. The end result in both pathways can be the same, that is, lysis of a foreign cell. Both pathways may also lead to opsonization or enhancement of inflammation. Binding of complement to antigen-antibody complexes can facilitate clearance of these potentially harmful masses by phagocytic cells.
Antibody bound to the surface of an invader may trigger contact killing of the invader by host cells in what is known as antibody-dependent, cell-mediated cytotoxicity (ADCC). Receptors for Fc of bound antibody on a microorganism or tumor cell cause natural killer cells to adhere to them and pour forth the cytotoxic contents of their vacuoles.
T-Cell Receptors T-cell receptors are transmembrane proteins on the surfaces of T cells. Like antibodies, T-cell receptors have a constant region and a variable region. The constant region extends slightly into the cytoplasm and the variable region, which binds with specific antigens, extends outward. Most T-cells also bear other transmembrane proteins closely linked to the T-cell receptors, which serve as accessory or coreceptor molecules. These are of one of two types: CD4 or CD8.
Subsets of T Cells
Lymphocytes are activated when they are stimulated to move from their recognition phase, in which they simply bind with particular antigens, to a phase in which they proliferate and differentiate into cells that function to eliminate the antigens. We also speak of activation of effector cells, such as macrophages, when they are stimulated to carry out their protective function.
Communication between cells in the immune response, regulation of the response, and certain effector functions are performed by different kinds of T cells. Although morphologically similar, subsets of T cells can be distinguished by characteristic proteins in their surface membranes. For example, cells with the coreceptor protein CD (for cluster of differentiation) are CD4+ and those with CD8 are described as CD8+. Until recently immunologists believed that certain CD4+ cells (T helper or TH) activated immune responses, and certain CD8+ cells (T suppressors) downregulated such responses. Present evidence now suggests a more complicated web of interactions (Figure 37-3). Some TH cells (designated TH1) activate cellmediated immunity while suppressing the humoral response, and others (called TH2) activate humoral and suppress cell-mediated immunity.
Cytotoxic T lymphocytes (CTLs) are CD8+ cells that kill target cells expressing certain antigens. A CTL binds tightly to the target cell and secretes a protein that causes pores to form in the cell membrane, resulting in lysis.
We discuss the role of MHC proteins in nonself recognition in the following text, but MHC proteins are not themselves the molecules that recognize foreign substances. This task falls to two basic types of molecules, the genes for which probably evolved from a common ancestor: antibodies and T-cell receptors. Each vertebrate individual has an enormous variety of antibodies, each of which binds specifically to one particular antigen (or part of the antigen), even though that antigen may have never been present in the body previously. There are probably an equal number of different T-cell receptors, each also specific for a particular antigen.
A major problem of immunology is understanding how the mammalian genome could contain the information needed to produce at least a million different antibodies. The answer seems to be that antibody genes occur in pieces, rather than as continuous stretches of DNA, and that the antigenrecognizing sites (variable regions) of the heavy and light chains of the antibody molecules are pieced together from information supplied by separate DNA sequences, which can be shuffled to increase the diversity of the gene products. The immense repertoire of antibodies is achieved in part by complex gene rearrangements and in part by frequent somatic mutations that produce additional variation in protein structure of the variable regions of the heavy and light antibody chains. Analogous processes occur in the production of genes for T-cell receptors.
Antibodies
Antibodies are proteins called immunoglobulins. They are borne in the surface of B lymphocytes or secreted by cells (plasma cells) derived from B cells. The basic antibody molecule consists of four polypeptide strands: two identical light chains and two identical heavy chains held together in a Y-shape by disulfide bonds and hydrogen bonds (Figure 37-2). The amino acid sequence toward the ends of the Y varies in both the heavy and light chains, according to the specific antibody molecule (the variable region), and this variation determines with which antigen the antibody can bind. Each of the ends of the Y forms a cleft that acts as the antigen-binding site (Figure 37-2), and specificity of the molecule depends on the shape of the cleft and properties of the chemical groups that line its walls. The remainder of the antibody is known as the constant region. The variable end of the antibody molecule is often called Fab, for antigen-binding fragment, and the constant end is known as Fc, for crystallizable fragment (Figure 37-2). The so-called constant region is not really constant: the light chains can be either of two types, and the heavy chains may be any of five types. The type of heavy chain determines the class of the antibodies, known as IgM, IgG (now familiar to many people as gamma globulin), IgA, IgD, and IgE. The class of the antibody determines the specific role of the antibody in the immune response (for example, whether the antibody is secreted or held on a cell surface) but not the antigen it recognizes.
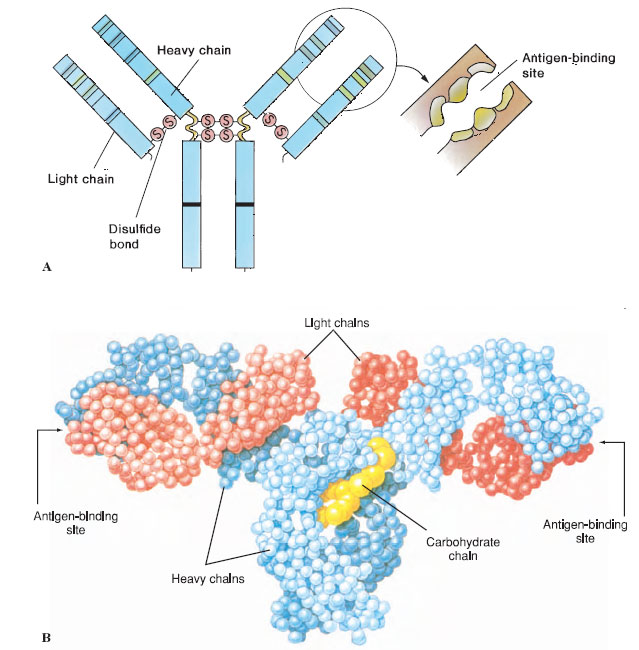 |
| Figure 37-2 A, Antibody molecule is composed of two shorter polypeptide chains (light chains) and two longer chains (heavy chains) held together by covalent disulfide bonds. These are further subdivided into variable and constant regions that have independent folding units, or domains, of about 110 amino acids. The folding pattern is more complex than that shown here. Interchain disulfide bonds at the hinge region give the molecule flexibility at that point. The variable domains of both the light and heavy chains have hypervariable ends, which serve as the antigen-binding sites. B, Molecular model of antibody molecule. |
Functions of Antibody in Host Defense Antibodies can mediate destruction of an invader (antigen) in a number of ways. A foreign particle, for example, becomes coated with antibody molecules as their Fab regions become bound to it. Macrophages recognize the projecting Fc regions and are stimulated to engulf the particle. This is the process of opsonization. Antibodies also may be able to neutralize toxins that are secreted by an invader.
Another important process, particularly in destruction of bacterial cells, is interaction with complement activated by the classical pathway. As noted previously, the first component in the classical pathway is activated by bound antibody. The end result in both pathways can be the same, that is, lysis of a foreign cell. Both pathways may also lead to opsonization or enhancement of inflammation. Binding of complement to antigen-antibody complexes can facilitate clearance of these potentially harmful masses by phagocytic cells.
Antibody bound to the surface of an invader may trigger contact killing of the invader by host cells in what is known as antibody-dependent, cell-mediated cytotoxicity (ADCC). Receptors for Fc of bound antibody on a microorganism or tumor cell cause natural killer cells to adhere to them and pour forth the cytotoxic contents of their vacuoles.
T-Cell Receptors T-cell receptors are transmembrane proteins on the surfaces of T cells. Like antibodies, T-cell receptors have a constant region and a variable region. The constant region extends slightly into the cytoplasm and the variable region, which binds with specific antigens, extends outward. Most T-cells also bear other transmembrane proteins closely linked to the T-cell receptors, which serve as accessory or coreceptor molecules. These are of one of two types: CD4 or CD8.
Subsets of T Cells
Lymphocytes are activated when they are stimulated to move from their recognition phase, in which they simply bind with particular antigens, to a phase in which they proliferate and differentiate into cells that function to eliminate the antigens. We also speak of activation of effector cells, such as macrophages, when they are stimulated to carry out their protective function.
Communication between cells in the immune response, regulation of the response, and certain effector functions are performed by different kinds of T cells. Although morphologically similar, subsets of T cells can be distinguished by characteristic proteins in their surface membranes. For example, cells with the coreceptor protein CD (for cluster of differentiation) are CD4+ and those with CD8 are described as CD8+. Until recently immunologists believed that certain CD4+ cells (T helper or TH) activated immune responses, and certain CD8+ cells (T suppressors) downregulated such responses. Present evidence now suggests a more complicated web of interactions (Figure 37-3). Some TH cells (designated TH1) activate cellmediated immunity while suppressing the humoral response, and others (called TH2) activate humoral and suppress cell-mediated immunity.
Cytotoxic T lymphocytes (CTLs) are CD8+ cells that kill target cells expressing certain antigens. A CTL binds tightly to the target cell and secretes a protein that causes pores to form in the cell membrane, resulting in lysis.




