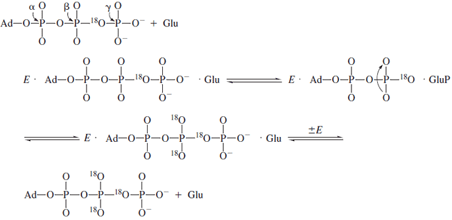Phosphate Transfer
Enzymes that catalyze the transfer of a phosphoryl moiety
between two substrates have provided excellent examples
of the use of isotopes in kinetic and stereochemical studies.
The enzyme hexokinase, which promotes the conversion
of glucose plus ATP to glucose-6-phosphate and
ADP has been the subject of kinetic studies that suggested
an ordered kinetic sequence with glucose being the first
substrate to add and glucose-6-P the last product to be released.
Specific information on the identity of rate-limiting
steps and the steady-state levels of reaction intermediates
was obtained by isotope trapping studies. In its simplest
form, enzyme and isotopically labeled substrate (
S*) are
incubated (the pulse) and rapidly diluted into excess unlabeled
substrate (the chase), and allowed to react for a
chosen time. Then the reaction is stopped by a quenching
reagent that jumps the pH or denatures the enzyme.
From the amount of
E ·
S* converted to product versus that
lost to dissociation (replacement by
S gives nonlabeled
product) the dissociation rate of
S* from
E and other
ES complexes can be calculated.
This method has been used in the study of the partitioning
of
ES complexes in the steady state. In the case
of hexokinase, the question was the partitioning of the
functional
E · glucose · ATP complex between product
formation and substrate release. For glucose the relevant
scheme is:
| |
E + Glc* |
koffGlc* |
E . Glc* |
koffATP |
|
kc |
E . Glc*-6-P . ADP |
koffADP |
E · Glc*-6-P + ADP |
→ E. |
| |
← |
← |
E . Glc* . ATP |
 |
→ |
| |
|
|
|
k−c |
|
In this case the reaction is allowed to reach steady-state
turnover, and the solution is either stopped by quench or
chased by addition of excess unlabeled substrate followed
by a delay sufficient for several turnovers then addition
of quench. The presence of a difference in the level of
the labeled product obtained by the two procedures represents
the concentration of E · Glc · ATP* complex in the
steady state, which is approximately 50% of ET, the total
enzyme concentration. The observed steady-state and pretransient
rates are consistent with steps k
c and k
−c being
at equilibrium relative to k
offADP, which is typical for many
phosphotransfer enzymes in which the chemical steps are
generally not rate limiting. Additional information can
be obtained by using the label in the second substrate
(i.e., [γ -
32P]ATP) and following a similar protocol, which
thereby allows calculation of the dissociation rate of ATP
from E · Glc · ATP. In this case E · Glc · ATP* is approximately
25% of ET, which requires that k
offATP compete with
the dissociation of ADP (k
offADP ) from E · Glc-6-P · ADP.
In this manner the individual rate constants for hexokinase
were largely determined and the order of substrate
association was verified.
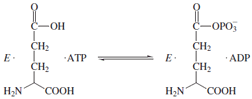
Isotopic labeling has also been cleverly used to demonstrate
the existence of enzyme-bound intermediates that
do not readily dissociate into solution. The enzyme glutamine
synthetase catalyzes the formation of glutamine
from ATP and ammonia possibly through a tightly bound
glutamyl phosphate intermediate.
If the formation of glutamyl phosphate were reversible
and occurred in the absence of ammonia, then the presence
of a symmetric torsion motion at the cleavage site might
be
used to detect an isotopic exchange brought about by glutamyl
phosphate formation. The synthesis of γ -18O
4P-ATP containing
an 18O label in the βγ bridging oxygen provides
the necessary probe for finding this intermediate
by means of the process below. In these experiments,
isotopes are used as labels so that the fate of a particular
atom may be followed throughout the course of the
reaction.
The appearance of
18O in the nonbridging oxygens of
the β-phosphate can be measured by mass spectrometric
and
NMR methods. The extent of equilibration is partially
inhibited by the presence of ammonia as required if glutamyl
phosphate is a reaction intermediate.
Isotopic labeling studies of phosphotranferase reactions
culminated in the synthesis of ATP chiral at the
γ -phosphorus. Chirality was achieved by the synthesis of
[γ -
16O,
17O,
18O]ATP of one configuration, and the analysis
of its chirality was achieved by stereochemically
controlled transfer of the γ -phosphoryl moiety to (
S)-
propane-1,2-diol where the absolute configuration was
determined by a chemical/mass spectrometric sequence.
The observation of inversion of configuration has been
accepted as evidence of an “in-line” displacement mechanism
at phosphorus by the two bound substrates; the observation
of retention of configuration was used to implicate
the existence of a phosphoryl enzyme intermediate in
the phosphoryl transfer process. For hexokinase, our case
study, the finding is one of inversion, consistent with a
direct transfer mechanism.