IDL and LDL Metabolism
Unlike chylomicron remnants, IDL has two competing metabolic fates: (1) uptake by the liver and (2) further processing to become LDL (Fig. 6).Since IDL can be cleared by the liver or can be processed to become LDL, this branch point represents an important stage where LDL concentrations can be regulated. Inefficient clearance of IDL tends to lead to increased LDL production.
LDL is formed in the bloodstream through the catabolism of VLDL at the surface of blood vessels. In addition, VLDL is a triglyceride-rich lipoprotein, while LDL is cholesterol rich. The production of a cholesterolrich lipoprotein from a triglyceride-rich lipoprotein occurs by selective removal of triglyceride from VLDL.
In summary, dietary fat is packaged into chylomicrons in the intestine. Dietary cholesterol is also packaged into chylomicrons, but a substantial fraction is delivered to the liver via chylomicron remnants. This cholesterol can then compete for the core of VLDL and appear in the bloodstream as cholesterol ester-enriched VLDL particles. Excess substrate in any form is converted into TG for export by the liver. For example, in some people, diets high in simple carbohydrates (e.g., fructose and sucrose) can lead to hypertriglyceridemia.
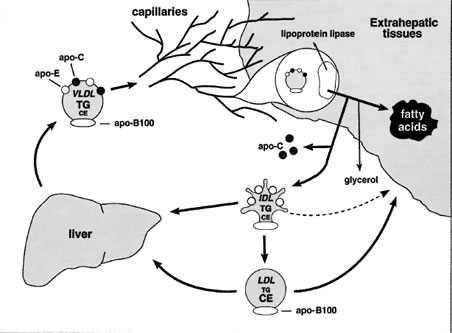 |
| Figure 6 The VLDL → IDL → LDL pathway. VLDL is secreted by the liver directly into the bloodstream. Like chylomicrons, VLDL triglyceride is a substrate for lipoprotein lipase and is hydrolyzed while at the luminal surface of adipose tissue and muscle capillaries. The VLDL remnant (IDL), unlike the chylomicron remnant, can give rise to LDL. Some IDL is also directly cleared by the liver. LDL is cleared by the liver and by extrahepatic tissues. |
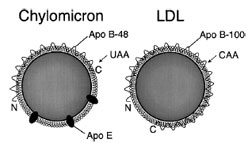 |
| Figure 7 Chylomicrons contain a different form of apoB than VLDL or LDL. In the intestine, an RNA editing event introduces a stop codon in apo-B, resulting in a truncated protein product, apo-B48. VLDL is secreted with full-length apoB, apo-B100, and thus gives rise to LDL particles with apo-B100. The receptorbinding domain of apoB is at the C-terminal half of the protein, thus apo-B48 cannot bind to the LDL receptor; chylomicrons depend upon apo-E for receptor binding. Note: the particles are not drawn to scale; chylomicrons are about five times larger than LDL particles. |




