Flavonoid Pigments
Hundreds of flavone-like pigments are widely distributed among plants. On the basis of their chemical structure, these pigments are grouped in several classes, the most important of which are listed in Table II. The basic structure of all these compounds comprises two benzene rings, A and B, connected by a heterocycle. The classification of flavonoids is based on the nature of the heterocycle (which is open in one class).Most of these pigments are yellow (Latin, flavus). One important exception is the anthocyanins, which display a great variety of red and blue hues. Because of the strong visual impact of anthocyanins on the marketing of fruits and vegetables, these pigments will be discussed in greater detail than other flavonoids.
1. Anthocyanins
The name of these pigments was originally coined to designate the blue (kyanos) pigments of flowers (anthos). It is now known that not only the blue color, but also the purple, violet, magenta, and most of the red hues of flowers, fruits, leaves, stems, and roots are attributable to pigments chemically similar to the original “flower blues.” Two exceptions are notable: tomatoes owe their red color to lycopene and red beets owe theirs to betanin, pigments not belonging to the anthocyanin group.
Anthocyanins are glycosides of anthocyanidins, the latter being polyhydroxyl and methoxyl derivatives of flavylium. The arrangement of the hydroxyl and methoxyl groups around the flavylium ion in six anthocyanidins common in foods is shown in Fig. 4.
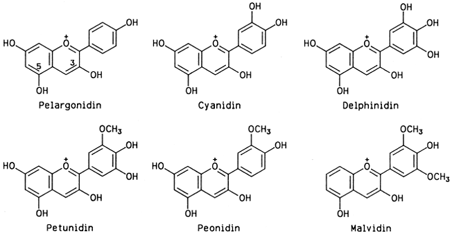 |
| Figure 4 Six anthocyanidins common in foods. The electric charge shown at position 1 is delocalized over the entire structure by resonance. |
There are at least 10 more anthocyanidins in nature, practically always appearing as glycosides. The number of anthocyanins far exceeds that of anthocyanidins, since monosaccharides, disaccharides, and at times trisaccharides glycosylate the anthocyanidins at various positions (always at 3, occasionally at 5, and seldom at other positions). Eventual acylation with p-coumaric, caffeic, and ferulic acids increases the number of natural anthocyanins. An example of acylated anthocyanin is the dark purple eggplant pigment delphinidin, 3-[4-(p-coumaroyl)-Lrhamnosyl-( 1→6)-D-glycosido] 5-D-glucoside.
The color of anthocyanins is influenced not only by structural features (hydroxylation, methoxylation, glycosylation, acylation), but also by the pH of the solution in which they are present, copigmentation, metal complexation and self-association.
The pH affects both the color and the structure of anthocyanins. In very acidic solution, anthocyanins are red, but as the pH rises the redness diminishes. In freshly prepared alkaline or neutral solution, anthocyanins are blue or violet, but (with the exception of certain multiacylated anthocyanins) they fade within hours or minutes.
In acidic solution four molecular species of anthocyanins exist in equilibrium: a bluish quinoidal (or quinonoidal) base A, a red flavylium cation AH+, a colorless carbinol pseudo-base B, and a colorless or yellowish chalcone C (Fig. 5).
![Four anthocyanin structures present in aqueous acidic solutions: R is usually H, OH, or OCH3. Gl is glycosyl. [Adapted from Brouillard, R. (1982). In “Anthocyanins as Food Colors” (P. Markakis, ed.), Academic Press, New York.]](images/3-5.gif) |
| Figure 5 Four anthocyanin structures present in aqueous acidic solutions: R is usually H, OH, or OCH3. Gl is glycosyl. [Adapted from Brouillard, R. (1982). In “Anthocyanins as Food Colors” (P. Markakis, ed.), Academic Press, New York.] |
![Absorption spectra recorded immediately after dissolving an anthocyanin (malvin chloride) in buffers of pH 2, 6, and 10. The absorption peaks at pH 6 and 10 disappeared within 1 to 3 hr. (Adapted from Brouillard, R. (1982). In “Anthocyanins as Food Colors” (P. Markakis, ed.), Academic Press, New York.]](images/3-6.gif) |
| Figure 6 Absorption spectra recorded immediately after dissolving an anthocyanin (malvin chloride) in buffers of pH 2, 6, and 10. The absorption peaks at pH 6 and 10 disappeared within 1 to 3 hr. (Adapted from Brouillard, R. (1982). In “Anthocyanins as Food Colors” (P. Markakis, ed.), Academic Press, New York.] |
At very low pH (below 1), the red cation AH+ dominates, but as the pH rises to 4 or 5, the concentration of the colorless form B increases rapidly at the expense of AH+, while forms A and C remain scarce. In neutral and alkaline solutions, the concentration of base A rises and its phenolic hydroxyls ionize, yielding unstable blue or violet quinoidal anions A− (Fig. 6).
TABLE II Major Classes of Flavonoids
| Class | Structure | Example (source) |
|---|---|---|
| Flavones | 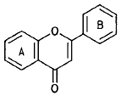 |
Apigenin (chamomile) |
| Flavan-3-ols (catechins) | 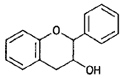 |
(—)-Epicatechin (cocoa) |
| Flavan-3, 4-diols | 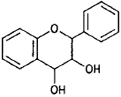 |
Leucocyanidin (peanut) |
| Flavanones | 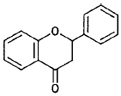 |
Naringenin, hesperidin (citrus fruits) |
| Flavonols | 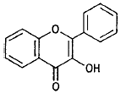 |
Quercetin (apples, grapes) |
| Flavanonols | 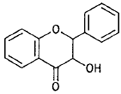 |
Taxifolin (Prunus) |
| Isofiavones | 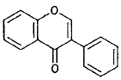 |
Genistein (soybeans) |
| Anthocyanidins | 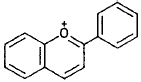 |
Pelargonidin, cyanidin, delphinidin (berries, red apples, red grapes) |
| Chalcones | 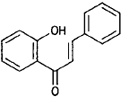 |
Butein (Butea) |
| Aurones | Aureusidin (Oxalis) |
Although it is true that the reaction of most plant tissues pigmented with anthocyanins (fruits, flowers, leaves) is slightly acidic, pH alone cannot explain the vivid colors encountered in these tissues. One mechanism leading to the enhancement and stability of anthocyanin coloration is copigmentation, that is, the association of anthocyanins with other organic substances (copigments). This association results in complexes that absorb more visible light (they are brighter) and light of lower frequency (they look bluer—the bathochromic effect) than the free anthocyanins at tissue pH. Most of these copigments are flavonoids, although compounds belonging to other groups (e.g., alkaloids, amino acids, nucleotides) can function similarly. A stacked molecular complex between an acylated anthocyanin and a copigment (flavocommelin) is shown in Fig. 7.
![Stacked molecular complex of awobanin and flavocommelin; p-C. denotes p-coumaroyl. [From Osawa, Y. (1982). In “Anthocyanins as Food Colors” (P. Markakis, ed.), Academic Press, New York.]](images/3-7.gif) |
| Figure 7 Stacked molecular complex of awobanin and flavocommelin; p-C. denotes p-coumaroyl. [From Osawa, Y. (1982). In “Anthocyanins as Food Colors” (P. Markakis, ed.), Academic Press, New York.] |
Self-association is the binding of anthocyanin molecules to one another. It has been observed that the complexes absorb more light than the sum of the single molecules. This explains why a 100-fold increase in the concentration of cyanidin 3,5-glucoside results in a 300- fold rise in absorbance.
Certain anthocyanins form complexes with metals (e.g., iron, aluminum, magnesium), and the result is an augmentation of the anthocyanin color. At times the complexes involve an anthocyanin, a copigment, and a metal.
Alarge number of the anthocyanins present in fruits and vegetables have been identified. It is not unusual for a plant tissue to contain several anthocyanins (17 in certain grape varieties), all genetically controlled. Table III shows the anthocyanidin moieties of anthocyanins in common fruits and vegetables.
TABLE III Anthocyanidins Present as Anthocyanins in Fruits and Vegetables
| Fruit or vegetable | Anthocyanidin |
|---|---|
| Apple (Malus pumila) | Cyanidin |
| Blackberry (Rubus fructicosus) | Cyanidin |
| Black currant (Ribes nigrum) | Cyanidin. delphinidin |
| Blueberry (lowbush,Vaccinium angustifolium; highbush,V. corymbosum) |
Delphinidin, petunidin,malvidin, peonidin,cyanidin |
| Cherry (sour, ‘Montmorency,’ Prunus
cerasus; sweet, ‘Bing,’ P. avium) |
Cyanidin, peonidin |
| Cranberry (Vacinnium macrocarpon) | Cyanidin, peonidin |
| Elderberry (Sambucus nigra) | Cyanidin |
| Fig (Ficus carica) | Cyanidin |
| Gooseberry (Ribes grossularia) | Cyanidin |
| Grape (red European. Vitis vinifera) | Malvidin, peonidin, delphinidin, cyanidin, petunidin, pelargonidin |
| Grape (‘Concord,’ Vitis labrusca) | Cyanidin, delphinidin, peonidin, malvidin, petunidin |
| Mango (Mangifera indica) | Peonidin |
| Mulberry (Morus nigra) | Cyanidin |
| Olive (Olea europea) | Cyanidin |
| Orange (‘Ruby,’ Citrus sinesis) | Cyanidin. delphinidin |
| Passion fruit (Passiflora edulis) | Delphinidin |
| Peach (Prunus persica) | Cyanidin |
| Pear (Pyrus communis) | Cyanidin |
| Plum (Prunus domestica) | Cyanidin, peonidin |
| Pomegranate (Punica granatum) | Delphinidin, cyanidin |
| Strawberry (Fragaria chiloensis and F. virginiaca) | Pelargonidin, little cyanidin |
| Beans (red, black; Phaseolus vulgaris) | Pelargonidin, cyanidin, delphinidin |
| Cabbage (red, Brassica oleracea) | Cyanidin |
| Corn (red, Zea mays) | Cyanidin, pelargonidin |
| Eggplant (Solanum melongena) | Delphinidin |
| Onion (Alium cepa) | Cyanidin, peonidin |
| Potato (Solanum tuberosum) | Pelargonidin, cyanidin, delphinidin, petunidin |
| Radish (Raphanus sativus) | Pelargonidin, cyanidin |
Generally, the attractive color of anthocyaninpigmented foods is not very stable. Canning of red cherries or berries results in products with considerable bleaching. Strawberry preserves lose one-half of their anthocyanin content after a few weeks on the shelf, although the browning reaction may mask the loss. And red grape juice is subject to extensive color deterioration during storage.
Exposure to high temperatures and contact with the oxygen of the air appear to be two factors affecting anthocyanin stability most adversely. Ascorbic acid accelerates the destruction of anthocyanins, and so does light. Certain oxidizing enzymes, such as phenol oxidase, and a hydrolyzing enzyme known as anthocyanase may contribute to the degradation of anthocyanin pigments. Oxidizing enzymes act on the anthocyanidin moiety, while anthocyanase splits off the sugar residue(s); the freed anthocyanidin is very unstable and loses its color spontaneously. Sulfur dioxide, which is used for the preservation of some fruit products (pulps, musts), bleaches anthocyanin pigments, but on heating of the fruit prduct in vacuum the SO2 is removed and the anthocyanin color reappears. Large concentrations of SO2, combined with lime, decolorize anthocyanins irreversibly and are used in the preparation of maraschino cherries. Anthocyanins act as anodic and cathodic depolarizers and thereby accelerate the internal corrosion of tin cans. It is therefore necessary to pack anthocyanin-colored products in cans lined with special enamel. In aging red wines anthocyanins condense with other flavonoids and form polymeric (MW ≤ 3000) redbrown pigments (Fig. 8). On continued polymerization these pigments become insoluble and form sediments in bottled red wines.
![Proposed structure for a polymeric red-brown pigment in aging red wine. [From Somers, T. C. (1971). Phytochemistry 10, 2184.]](images/3-8.gif) |
| Figure 8 Proposed structure for a polymeric red-brown pigment in aging red wine. [From Somers, T. C. (1971). Phytochemistry 10, 2184.] |
Anthocyanins possessing more than one acyl group show extraordinary color stability over a wide pH range. One of them, peonidin-3-(dicaffeyl sophoroside) 5-glucoside, isolated from ‘Heavenly Blue’ morning glory flowers (Ipomoea tricolor), has been shown to “produce a wide range of stable colors in foods and beverages which have a pH range of 2.0 to about 8.0.” United States patent 4,172,902 covers its use as a colorant in foods.
2. Other Flavonoids
Among flavonoids other than anthocyanins, the catechins, flavonols, and leucoanthocyanidins have the widest distribution in foodstuffs, while flavonone glycosides are of special interest in citrus fruits.
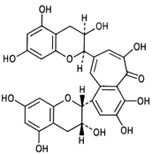 |
| Figure 9 Structure of the aflavin. |
Flavonols, like anthocyanidins, exist almost exclusively as glycosides. Three common flavonols are kaempferol, quercetin, and myricetin, resembling pelargonidin, cyanidin, and delphinidin, respectively, in the hydroxylation pattern of theBring. Flavonol glycosides impart weak yellow hues to apples, apricots, cherries, cranberries, grapes, onions, plums, potatoes, strawberries, tea, tomatoes, and other commodities.
Leucoanthocyanidins are compounds of the general formula
- shown in Fig. 10. They have no color of their own, but in acidic environments and at elevated temperatures they are converted to colored anthocyanidins.
- This reaction is in competition with the condensation to a dimeric leucoanthocyanidin.
- Low temperature favors the formation of the dimeric compound, which can polymerize to yield products with pronounced tanning properties.
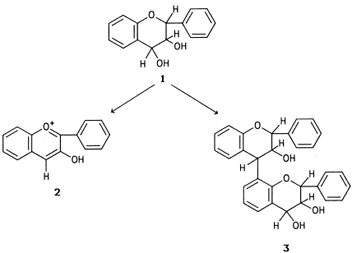 |
| Figure 10 Basic structures of leucoanthocyanidins (1), anthocyanidins (2), and dimeric leucoanthocyanidins (3). |
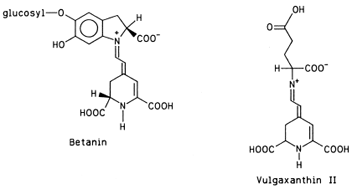 |
| Figure 11 Two major pigments of red beets. |




