Microslide Preparation of Metaphases for In-Situ Hybridization
PurposeMetaphases are prepared from lymphocytes for in-situ hybridization experiments. The lymphocytes, already uspended in fixative, are dropped from a given height onto cleaned slides. The best preparations of metaphases are obtained when the cells are dropped onto slides immediately after the last wash of the harvesting procedure. The lides are then air-dried, labeled, rinsed in alcohol, and stored in slide boxes at –80°C
Time Required
In 3–4 hours, approximately 80–100 microslides can be prepared for in-situ hybridization.
Materials
- Microslides, 25 × 75 mm
- Reagent absolute alcohol
- Solution A or phosphate-buffered saline
- Diamond scriber
- Microslide box, for 100 slides
- Coplin staining dishes (4)
- 9" × 9" Technicloth wipes
- Forceps
- Nikon TMS inverted microscope (Frank E. Fryer Company) equipped with 20X and 40X phase contrast objectives and a green filter. The microscope is also equipped with L20 and L40 phase annulus for the LWD condenser.
Procedure
- Presoak 6–8 microslides (slides) in absolute alcohol in a coplin staining dish (coplin jar) for several minutes.
- Aspirate the old fixative above the cell pellet and resuspend the pellet in 1.5 mL of “fresh” fixative.
- Using forceps, remove 1 slide from the coplin jar and wipe dry with a 9" × 9" wipe.
- Scratch 2 parallel lines approximately 22-mm apart on the lower third of
unfrosted “back” side of slide:
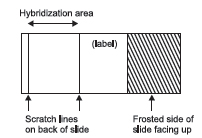
The scratches will define the area of hybridization of probe DNA and metaphase DNA.
The room temperature, the barometric pressure, and the humidity will affect the drying time of cells and the quality of the metaphases. It is essential to try to control all the variables and strive to achieve the optimum conditions. The use of vaporizers on days when the air is extremely dry and air conditioners when the room temperature is above 18°C, will help to produce high quality metaphases. Optimum conditions are: room temperature between 15°C–18°C, >50% humidity (a rainy day) and low barometric pressure. - Drop 100 µL of the cell suspension from 3-4" above the slide surface onto the frosted side of the slide. Drop the suspension slowly, 1 drop at a time, moving the pipetman so that no 2 drops fall exactly on the same surface area (but all 100 µL should fall within the scratched area on slide). The fixative will quickly evaporate from under and around the cell and the cell will flatten completely, forcing the chromosomes to spread. Different methods of making slides may speed up or slow down this evaporative process, causing more or less spreading. Throughout the whole process, the cell membrane is present. If a cell breaks open during any stage of the harvest or slide making, some or all of the chromosomes will spill out, causing hypodiploidy with random loss of chromosomes. In severe cases, the slides will have “scattered” chromosomes or completely lack chromosome spreads. To shorten the evaporation time and prevent “scattering” of chromosomes, hold the slide at a 45° angle. Well-spread chromosomes will also have less cytoplasm around each chromosome.
- Air-dry the slide either at a slant if the chromosomes are well spread, or horizontal if the chromosomes are not well spread. Quickly scan every slide, using the 20X objective, green filter, and L20 phase annulus. Be careful when viewing the slides; scratching the surface will ruin chromosome material. Check to see if the chromosomes are spread sufficiently and that no cytoplasm is observed (halo or dark area around chromosomes). If cytoplasm is detected, disperse it by washing the cells several more times with “fresh” fixative before preparing more slides. Also, check to see if the suspension is too concentrated or too dilute. Concentrated cell suspensions will produce underspread metaphases, while dilute suspensions are very time-consuming to scan for metaphases.
- In a coplin jar, wash 5 or 6 slides at a time in 50 mL of PBS for 5 minutes. Always be careful not to scratch the slides.
- After the slides are prepared, wash them in a series of ethanol rinses, 5 minutes each, to rid slides of remaining acetic acid. This is done by increasing the percentage of ethanol in each wash (70, 90, 100%). The chromosomes will harden and become more resistant to conventional banding procedures, e.g., G or Giemsa banding. Therefore, it is important not to wash the slides after slide preparations are done if they will be used for procedures other than in-situ hybridization.
- Air-dry the slides vertically for several minutes and transfer them to a
microslide box. Wrap parafilm around the box prior to freezing the slides
at –80°C (prevents moisture from entering). Slides can be stored for 1 year
at –80°C.
Do not store slides at –20°C or at +4°C, because the quality of the chromosomes
deteriorates.




