Insertion of a Foreign DNA Fragment into a Vector

Fig. 4.1. Diagrammatic presentation of gene cloning.
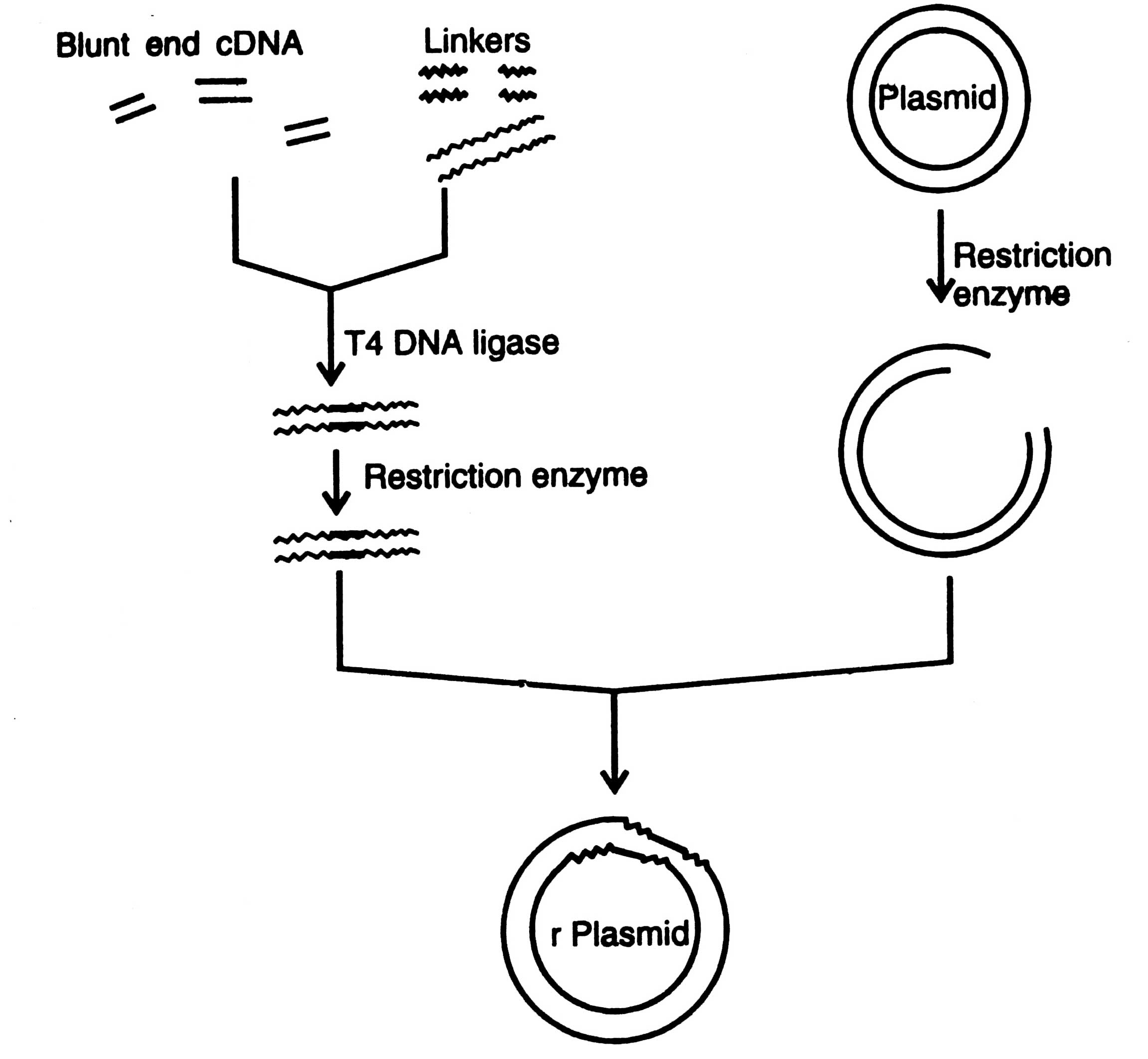
Fig. 4.2. Insertion of a foreign DNA fragment into a plasmid by using restriction enzyme linkers.
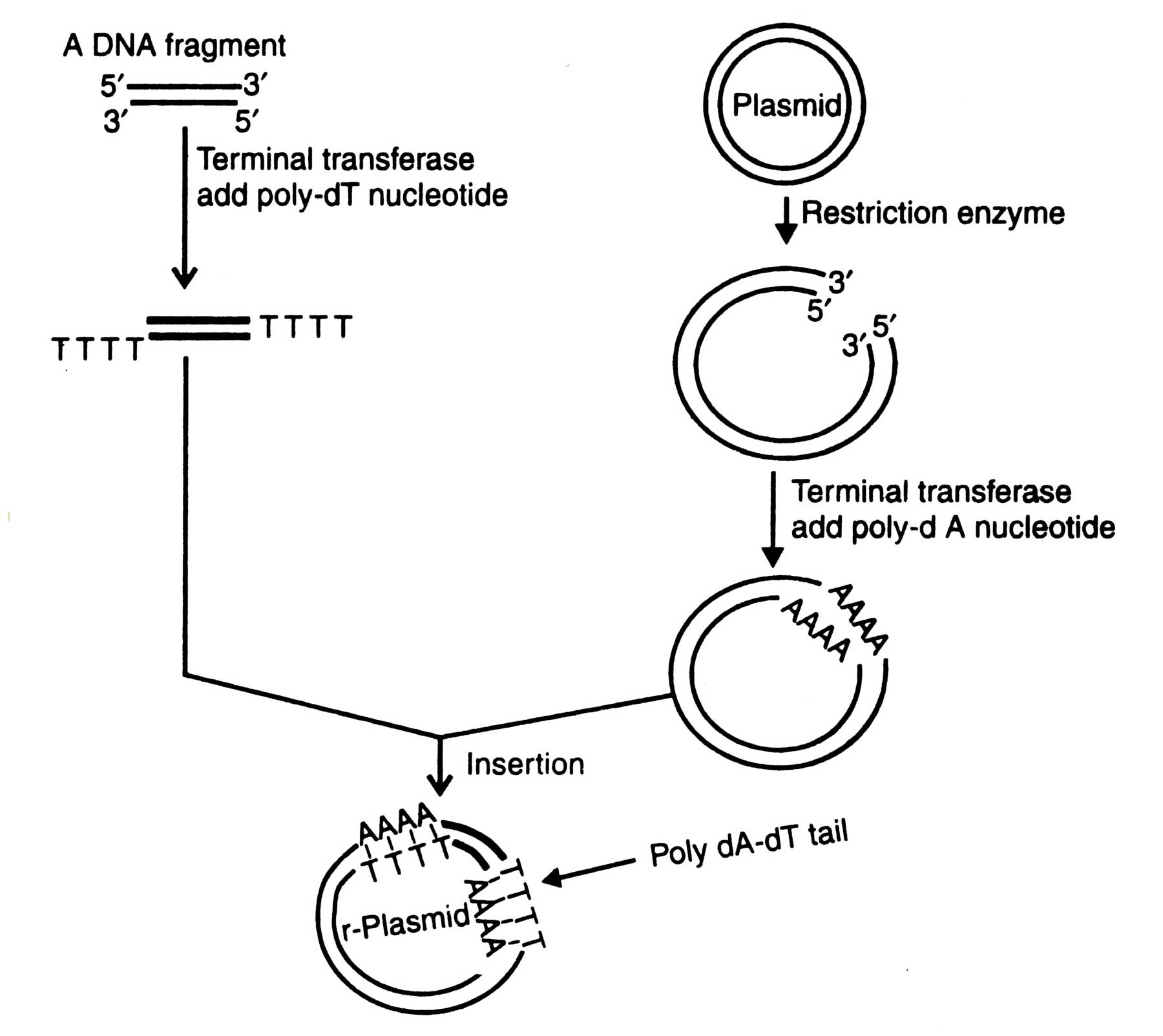
Fig. 4.3. Insertion of a foreign DNA fragment into a plasmid by using homopolymer tail.
For insertion of double stranded cDNA into a cloning vector it is necessary to add to both termini single stranded DNA sequence which should be complementary to a tract of DNA at the termini of linearlized vector. In order to get efficient formation of recombinant DNA molecules, addition of sticky ends on both termini is necessary. There are two methods for generation of cohesive ends on the double stranded cDNA, use of linkers and homopolymer tails.
Use of restriction enzyme linkers
Linkers are the chemically synthesized double stranded DNA oligonucleotides containing on it one or more restriction sites for cleavage by restriction enzymes, e.g. Eco RI, Hind III, Bam HI, etc. Linkers are ligated to blunt end DNA by using T4 DNA ligase (Fig. 4.2). Both the vector and DNA are treated with restriction enzyme to develop sticky ends. The staggered cuts i.e. sticky ends are then ligated with T4 DNA ligase with very high efficiency to the termini of the vector and recombinant plasmid DNA (chimeric DNA) molecules are produced.
Use of homopolymer tails
Using terminal transferase the synthesis of homopolymer tails of the defined length at both 3' termini of double stranded DNA and vector is possible. In the presence of precursor dATP, terminal transferase helps to add poly-dA at 3' termini of vector. Likewise the same enzyme adds poly-T at 3' termini of DNA molecule, when precursor TTP is present. The linearized vector having tails is incapable of recircularization, unless ligated to a double stranded DNA fragment. The vector and DNA tails are annealed. The poly dA-dT tails are then ligated by T4 DNA ligase (Fig. 4.3). If poly dG-dC tails are used, instead of poly dA-dT tails, a high initiation temperature (upto 37°C) is required for the annealing reaction. The major advantage in using poly dG-dC tails is that the hybrids are more stable than poly dA-dT hybrids (Williams, 1981).
In the first method, the inserted DNA molecule can be retrieved by using restriction enzymes (e.g. EcoRl) on the cleavage sites of linker, whereas in the second method, however, it is very difficult to retrieve the inserted DNA fragment due to the loss of recognition sites when poly dA-dT tails are added. In the second case, recovery of the inserted DNA fragment is possible only when poly dG-dC tails are used, because it gives recognition site for restriction enzymes, for example PstI.




